Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.9 no.1 Ciudad de México Abr. 2010
Ingeniería de alimentos
In vitro lead and nickel accumulation in mesquite (Prosopis laevigata) seedlings
Acumulación in vitro de plomo y níquel en plántulas de mezquite (Prosopis laevigata)
L. Buendía-González1,2, J. Orozco-Villafuerte2*, M. E. Estrada-Zúñiga1, C. E. Barrera Díaz2, E. J. Vernon-Carter3 and F. Cruz-Sosa1
1 Departamento de Biotecnología, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco No. 186, Col. Vicentina, Iztapalapa, C.P. 09340, México, D.F. México.
2 Facultad de Química, Universidad Autónoma del Estado de México, Paseo Colón esq. Paseo Tollocan s/n, Col. Residencial Colón, C.P. 50120 Toluca, Estado de México, México. *Corresponding author. E-mail: jov202001@yahoo.com.mx Tel. (01722) 217 5109 Fax (01722) 217 3890.
3 Departamento de lngeniería de Procesos e Hidráulica, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco 186, Col. Vicentina, Iztapalapa, C.P. 09340 México, DF, México.
Received 19 of November 2009;
Accepted 18 of December 2009
Abstract
The growth, survival and Pb(II) and Ni(II) uptake of Prosopis laevigata seedlings were determined in order to evaluate their bioaccumulation capability. The seedlings were cultured during 50 days on modified Murashige and Skoog medium supplemented with 10 g.l-1 of sucrose, 0.0, 0.32, 0.63, 1.26, 2.10, 4.20 mM Ni(II), and 0.0, 0.23, 0.45, 0.90, 1.50, 3.0 mM Pb(II). None of the studied heavy metals avoided germination; however, both produced smaller plants with fewer leaves and secondary roots. Seedlings showed an accumulation of 2 582 and 3 895 mg Ni kg-1, and of 27 300 and 40 666 mg Pb kg-1, both in dry basis (d.b.), in shoot and root, when cultured with 1.26 mM Ni and 3.0 mM Pb, respectively. These results indicated that significant translocation from the roots unto aerial parts took place. A bioaccumulation factor for Ni superior to 32 and for Pb over 21 was exhibited by the seedlings, so that P. laevigata can be considered as a viable accumulator species of Pb(II) and Ni(II) for phytoremediation purposes.
Keywords: Prosopis laevigata, phytoremediation, bioaccumulation, heavy metals, in vitro culture.
Resumen
Se determinó el crecimiento, sobrevivencia y acumulación de Pb (II) y Ni (II) en plántulas de Prosopis laevigata, con el fin de evaluar su capacidad de bioacumulación. Las plantas fueron cultivadas durante 50 días en medio de cultivo Murashige y Skoog suplementado con 10 g.l-1 de sacarosa, 0,0, 0,32, 0,63, 1,26, 2,10, 4,20 mM de Ni(II), y 0,0, 0,23, 0,45, 0,90, 1,50, 3,0 mM de Pb(II). Ninguno de los metales pesados estudiados evitó la germinación, sin embargo, ambos metales produjeron plántulas más pequeñas, con menos hojas y raíces secundarias. Las plántulas mostraron una acumulación en tallos y raíces de 2 582 y 3 895 mg Ni kg-1 y 27 300 y 40 666 mg Pb kg-1, en base seca (b.s.), cuando son cultivadas con 1,26 mM de Ni y 3,0 mM de Pb, respectivamente. Estos resultados indican que una significativa translocación de las raíces a las partes aéreas se llevó a cabo. Un factor de bioacumulación para Ni superior a 32 y por arriba de 21 para Pb fue determinada para las plántulas, de modo que P. laevigata puede considerarse como una prometedora especie acumuladora de Pb (II) y Ni (II) para fines de fitorremediación.
Palabras clave: Prosopis laevigata, fitorremediación, bioacumulación, metales pesados, cultivo in vitro.
1. Introduction
Ever since the Iron Age began, human beings have been polluting the environment with heavy metals as their populations have increased. These practices have considerably magnified, particularly following the industrial revolution. Even unsuspected materials may contain toxic metals such as commercial fruits and grains grown with polluted fertilizers (Saier and Trevors, 2008). One of the most toxic heavy metals is lead (Pb). Pb is an extremely toxic heavy metal which is a serious threat to the health of children and wildlife (EPA, 2009). The main sources of Pb poisoning include lead paint and old gasoline spills, although other major sources with high Pb concentrations are Pb mines and smelters, shooting ranges, and disposal sites for old batteries (Peer et al., 2005; Xintaras, 1992). Another threatening heavy metal is nickel (Ni), an essential element that can be toxic and possibly carcinogenic in high concentrations (ATSDR, 2005) Ni toxicity in humans usually results from repeated occupational exposure resulting in dermatitis, asthma or headaches (Akkenson and Skerfing, 1985; Davies, 1986), but Ni contamination of soils is primarily restricted to regions surrounding Ni smelting operations (Lupankwa et al., 2004; Johnson and Hale, 2004). Ni mining and smelting wastes often contain other metals such as Pb, and frequently pollute soil and water (Lupankwa et al., 2004).
Nowadays soil contamination with heavy metals is a world wide problem; areas with anthropogenic pressures often exceed governmental safety limits leading to significant losses in agricultural yield and hazardous health effects (Frankenberger, 2002; Jonak et al., 2004; Lupankwa et al., 2004). Even today, the most commonly used methods of dealing with heavy-metal pollution are ineffectual burial or isolation processes of the polluted land, which are also extremely costly operations. An ongoing research topic is to develop promising methods for restoring polluted sites. One such method is related to characteristics of plant species, which are capable to accumulate or tolerate unusually high concentrations of metals such as Pb and Ni (Kumar et al., 1995). In phytoremediation plants are used to reduce, remove, degrade, or immobilize environmental toxins, primarily those of anthropogenic origin, with the aim of restoring polluted sites to a condition usable for private or public applications. This technology is relatively inexpensive and aesthetically pleasing to the public compared to alternative remediation strategies (Peer et al., 2005).
Extensive phytoremediation research has been carried out with in vitro cultures that examine the intrinsic toxicity tolerance and accumulation metabolic capabilities of plant cells. The ability to identify the contributions of plant cells to pollutant uptake and detoxification without interference from microorganisms is of particular significance in the search for fundamental knowledge about plants (Doran, 2009). The aim of this work was to investigate the in vitro ability of Prosopis laevigata (mesquite) seedlings to remove two different heavy metals in different concentrations from the culture media, and to assess the effect of these metals uptake in the growth, morphology and survival of the plant.
2. Material and methods
2.1. Plant material
Mature brown pods were collected from adult Prosopis laevigata (Humb. and Bonpl. ex Willd M.C. Johnston) trees growing naturally in the Mexican State of San Luis Potosi. Mature seeds were isolated from the pods and were scarified mechanically. Under laminar flow hood they were disinfested by immersion in ethanol, followed by immersion in sodium hypochlorite (Buendía-González et al., 2007). Seeds were carefully rinsed with sterile bi-distilled water five times and germinated aseptically in culture tubes (25 x 150 mm) containing 15 ml of modified Murashige and Skoog medium (MS) (Murashige and Skoog, 1962). Filter paper (10.1 cm x 1.5 cm, Whatman 1004 917) segments were put inside the culture tubes for the purpose of supporting the seeds.
2.2. Heavy metals tested
The heavy metal (HM) sources used in this study included Pb(NO3)2 and NiCl2·6H2O salts (Baker Analyzed, Phillipsburg, NJ). Stock solutions of each heavy metal salt were prepared at a concentration of 10 g l-1 .
2.3. Media preparation and culture conditions
The modified MS medium was prepared as follows: (1) some salts contained in the original MS medium are prone to react violently or form precipitates when entering in contact with the heavy metals, and therefore these were eliminated and the medium composition was formulated according to Buendía-González et al. (2010); (2) 10 g l-1 sucrose; and (3) aliquots of the heavy metal salt stock solutions were added in order to achieve HM concentrations of 0.00, 0.23, 0.45, 0.90, 1.50, 3.0 mM of Pb and 0.00, 0.32, 0.63, 1.26, 2.10, 4.20 mM of Ni in the culture media. All media were adjusted to pH 5.8 with 1N NaOH and 1N HCl before autoclaving at 121ºC for 18 min. All cultures were maintained at 25º ± 2ºC under warm-white fluorescent light at an irradiance of 50 mmol m-2 s-1 and a 16 h (light) 8 h-1 (dark) photoperiod.
2.4. Evaluation of plant growth and heavy metal resistance
One seed was aseptically placed into each culture tube. Percentage of seed germination was registered after 10 days of culture. Morphological observations and growth seedling measurements were made at 10-days intervals up to 50 days. The root and shoot length of the seedlings inside the sealed culture tubes were measured. Seedling length was measured from the main root apex to the main shoot apex. The survival response was evaluated as the percentage of surviving plants from five seeds planted at the end of 50 days of culture. For growth measurement purposes surviving seedlings were harvested after 50 days, rinsed with deionized water three times and dried in a convection oven at 60°C for 72 hours. Their weight was determined and reported on a dry basis (d.b), and this value was considered as the plant biomass.
Growth measurements were used for evaluating the growth ratio (GR) and the heavy metal tolerance index (TI) resistance indicators, which are defined as (Baker, 1987):

2.5. Analysis of lead and nickel contents in biomass
50 days-old surviving seedlings were separated into root and aerial parts and were dried as indicated in the previous section. Dried tissue was weighed, powdered and digested with 5 ml of concentrated HNO3 in a microwave oven (CEM Mars5, CEM corporation, Mathews, NC), and finally the final sample volume was adjusted to 10 ml with deionized water and placed in HDPE flasks. The metals concentration was analyzed from the samples using a Varian Spectra AA-220 FS Atomic Absorption Spectrometer (Varian, Australia). All glassware and apparatus were washed with 0.1 N HNO3 before use.
Metal concentration measurements were used for evaluating the bioaccumulation factor (BF) and translocation factor (TF), which are defined as (Niu et al., 2007; Zhao et al., 2003):

2.6. Statistical analysis
All the experiments were carried out in quintuplicate with three replicates. All the experimental data obtained in this work were subjected to an analysis of variance (ANOVA) by using the NCSS version 5 statistical software (Wireframe Graphics, Kaysville, UT). Comparisons between means were carried out by using Tukey's multiple range test at the 5 % level of probability.
3. Results
3.1. Heavy metals effect on germination, survival response, and plant growth
P. laevigata seeds showed germination of 100 % irrespectively of the Pb(II) and Ni(II) concentration used. However, the morphologic characteristics of the seedlings were affected by both metals, manifested in a smaller size, fewer leaves, and fewer secondary roots in the seedlings compared to the control treatment (without HM) (Fig. 1). Nickel had a more pronounced effect on these seed-ling characteristics than lead. In the HM and control treatments, plants germinated after 24 h, so that germination time was not affected by HM.

The survival response of P. laevigata indicated that some of the Ni(II)-treated seedlings (2.10, 4.20 mM) showed a significantly lower survival percentages than the Pb(II)-treated seedlings (0.23, 0.45, 0.90, 1.50, 3.0 mM) (Fig. 2).

The effects of Ni(II) and Pb(II) concentrations on shoot and root length in P. laevigata seedlings are shown in Fig. 3. In general terms, data show a reduction in shoot and root length as HM concentrations increased. The effect was more pronounced on root than in shoot length reduction. The shoot length was reduced by 17.02 %, 23.40 %, 45.04%, 57.98% and 73.05% when exposed to 0.32, 0.63, 1.26, 2.10 and 4.20 mM Ni(II), and of 8.51%, 13.48%, 12.77%, 13.48% and 23.76%, when put in contact with 0.23, 0.45, 0.90, 1.50 and 3.0 mM Pb(II) compared to the control shoot length. The root length was reduced by 58.50%, 69.70%, 75.55%, 84.90% and 92.6% when exposed to 0.32, 0.63, 1.26, 2.10 and 4.20 mM Ni(II), whereas it was 59.70 %, 65.60 %, 65.60 %, 67.90 % and 78.20 %, when exposed to 0.23, 0.45, 0.90, 1.50 and 3.0 mM Pb(II) compared to the control root length.

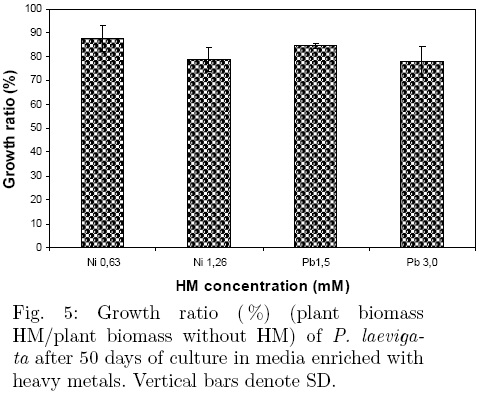
P. laevigata seedlings resistance had lower tolerance index (TI) values in Ni(II) treated seedlings than in the Pb(II) treated plants (Fig. 4). The TI was significantly greater for the seedlings treated with a 0.32 mM Ni(II) or 0.23, 0.45, 0.90 and 1.50 mM Pb(II) concentrations regarding the rest of metal concentration tested. Another way of measuring the resistance of seedlings to heavy metals is by determining the growth ratio (GR). The GR of P. laevigata was evaluated at two concentrations of Ni(II) (0.63 and 1.26 mM) and of Pb(II) (1.5 and 3.0 mM), respectively. The GR decreased as Ni(II) and Pb(II) concentration increased (Fig. 5). A dry biomass weight reduction in the seedlings of 12.33 and of 21.10 % occurred when Ni(II) concentration was increased from 0.63 to 1.26 mM, and of 15.41 and 22.28% when Pb(II) concentration was increased from 1.5 to 3.0 mM compared to the control, but these reductions were non-statistically different among themselves. Because some seedlings died before the 50 days harvesting time, mainly at the two highest Ni concentrations tested, neither their GR nor heavy metal uptake was determined.

3.2. Heavy metal uptake
The seedlings accumulated 2 430 and 3 895 mg of Ni kg-1 (d.b.) in root and 1 925 and 2 582 mg Ni kg- 1 (d.b.) in shoot when treated with 0.63 and 1.26 mM Ni(II), and 37 857 and 40 666 mg of Pb kg- 1 (d.b.) in root and 6 660 and 27 300 mg Pb kg- 1 (d.b.) in shoot when treated with 1.5 and 3.0 mM Pb(II) concentrations, respectively (Fig. 6). P. laevigata bioaccumulation factor (BF) decreased (from 52 to 32) as Ni(II) concentration increased from 0.63 to 1.26 mM, but BF increased (from 21 to 43) as Pb(II) concentration increased from 1.5 to 3.0 mM, respectively (Fig. 7). Translocation factor (TF) showed a similar trend as that exhibited b BF for the Ni(II) and Pb (II)-treated seedlings. The Ni(II)-treated seedlings showed a TF decrease from 0.79 to 0.61 as Ni(II) concentrations increased from 0.63 to 1.26 mM Ni(II), whereas the Pb(II)-treated seedlings showed an increment in TF from 0.17 to 0.67 for Pb(II) concentrations of 1.5 and 3.0 mM, respectively (Fig. 8). These TF values showed that mesquite was more efficient for translocating Ni than Pb. A significantly higher TF value was found for the 0.63 mM Ni(II) treatment.



4. Discussion
In this work the MS culture media was modified in order minimize the effect of certain factors that have been reported as hindering the bioavailability of the HM. Thus, we used a liquid medium instead of semi-solid medium because it has been reported that the addition of gelrite or agars ma increase the Ni content in the culture medium in a range of 0.004 -0.045 mmol kg-1 (George and Klerk, 2008). The addition of some salts originally contained in the MS culture medium formulation was avoided because the can form precipitates with HM making Pb unavailable. Also the inclusion of ED-TA was avoided because it increases the Pb and Ni availability , and the estimation of these HMs may be overestimated. Efforts of Pb phytoremediation have concentrated on using soil amendments like EDTA to increase the Pb availability, so that its uptake can be possible (Blaylock et al., 1997; Huang et al., 1997; Wu et al., 1999). Although the addition of chelators increases the Pb solubility and uptake, the amount of Pb transferred to shoots is still low in comparison to the Pb soil amount. Then it increases the likelihood that Pb-EDTA be mobilized and leached out, causing the groundwater contamination.
The toxic effect of polluting agents on the germination of seeds is a good indicative of possible tolerance of the plant against these contaminants (Carrillo-Castañeda et al., 2002), so that is important to study the effect of HM on seeds response. Our P. laevigata seeds showed germination of 100 %. Although both metals showed a toxic effect in seedlings, nickel had a more pronounced effect on these seedling characteristics than lead. A study with Medicago sativa using various heavy metals including nickel in a concentration of 10-40 mg l-1 (~0.17-0.68 mM Ni) (Peralta et al., 2001) produced a reduction in seed germination equivalent to lower doses used in this study. Studies by Salvatore et al. (2008) showed that at various levels of Ni(II) and Pb(II) in agar or filter paper (0.002-1.024 mM) did not have an effect on the germination of lettuce, broccoli, tomato and radish.
The survival response of P. laevigata indicated that the Ni(II)-treated seedlings showed a significantly earlier death time and significantly lower survival percentages than the Pb(II)-treated seedlings. These data demonstrated that the mesquite is more tolerant to Pb(II) than to Ni(II) toxicity. Moreover, this effect was corroborated when shoot and root length measurements were carried out. Seedlings showed a reduction in shoot and root length as HM concentrations increased, with a more pronounced deleterious effect on seedlings grown in medium supplied with Ni(II) than with Pb(II). A study with alfalfa seedlings exposed 500 mg l-1 (~2.10 mM) Ni(II) showed a lethal effect on seedlings growth after 16 days (Peralta-Videa et al., 2004). Salvatore et al. (2008) reported that a reduced root length in lettuce, broccoli, tomato and radish occurred when they were exposed to lead and nickel, among other heavy metals. Furthermore, they found that HM inhibition effect on root length occurred at lower concentrations when agar rather than filter paper was used as support. Kukier and Chaney (2004) suggested that if heavy metals are not rapidly detoxified, they may trigger multiple deteriorative events in plants by altering major physiological and biochemical processes, ultimately leading to visible plant injuries and yield losses.
Measurement of the seedling resistance to heavy metals is were carried out by determining the TI and the GR. P. laevigata seedlings resistance measurement showed that mesquite was more tolerant to lead than nickel; TI was higher in Pb(II)-treated seedlings by a 2.9 times factor than in the Ni(II)-treated seedlings, but GR was non-significantly different. The significant higher toxic effect of nickel in the mesquite seedlings could be due to, the intrinsic toxicity of the heavy metal plus, the fact that the culture medium provided only one source of nitrogen (NH4NO3), which is an essential element for the plant metabolism. Nitrogen is a constituent of proteins and nucleic acids, and also occurs in chlorophyll. Deficiency of nitrogen results in a loss of vigor, and the leaves showed symptoms of chlorosis which resulted in a reduced the number of them in whole plants (George and Klerk, 2008). On the other hand, the lead salt source (Pb(NO3)2) also contained nitrogen.
The Pb(II)-treated seedlings accumulated more metal than the Ni(II)-treated seedlings in root and shoot tissue. Results showed that the averages of Pb and Ni found in the root and shoot tissues were significantly different. They suggest that HM concentration tended to affect the Pb(II) and Ni(II) uptake capacities of the mesquite tissue seedlings. The uptake results from Pb(II)-treated P. laevigata seedlings showed similar results with Prosopis spp. at 75 mg l-1 of Pb ( 0.36 mM) in a hydroponic culture, which showed a lead uptake of 63 396, 1 417, and 51 mg Pb kg-1 (d.b.) in root, stem and leaf, respectively (Aldrich et al., 2004). P. laevigata accumulated more Pb than Ni, and the results of this work clearly indicated that Ni(II) was more toxic than Pb(II), causing more damage which resulted in a lower growth and survival of the seedlings. Species capable of accumulating metals at levels 100-fold or higher in the shoots with regard to common non-accumulator plants are considered as hyper-accumulators. Ideally, these plants should accumulate metals at levels up to 0.1-1.0% of the dry weight plant biomass produced, depending on the specific metal being taken up (Pilon-Smits, 2005). Thus, a hyperaccumulator will concentrate more than 1000 mg Pb or Ni kg-1 (d.b.) (Baker et al., 2000). According to HM accumulation results, P. laevigata might be considered a hyperaccumulator species of both heavy metals. At least 317 nickel-hyperaccumulating taxa are now known (Baker etal. , 2000). Hyperaccumulation of lead is particularly rare. The low solubility of most lead compounds in circumneutral media, and the ready precipitation of lead by sulphate and phosphate at the root systems may partly explain this (Baker et al., 2000). Thlaspi rotundifolium ssp. cepaerfolim grown in lead/zinc mining area of northern Italy achieved a lead accumulation up to 8 200 mg kg-1 (d.b.) (Reeves and Brooks, 1983). Alyssum wulfenianum Schelecht from the same location also contained remarkably high Pb concentrations, reaching values of 860 mg kg-1 (d.b.) in leaf.
Metal concentrations in shoot tissues are generally the standard used for defining a hyperaccumulator but the accumulation factor is highly recommended for understanding the feasibility of phytoextraction. The BF is more important than shoot concentration for considering the potential of phytoextraction for a given species. This work showed a BF of above 32 for Ni(II) and over 21 for Pb(II) for P. laevigata. In metal excluder species, the BF is typically lower than 1, whereas in metal accumulator species the factor is often greater than 1 (Baker, 1981).
Transport across root cellular membrane is an import process which initiates metal absorption into plant tissues. For the phytoextraction process, substantial amounts of the HM must be removed by the root from the medium, followed by their translocation to the harvestable plant parts, so that they can be completely removed from the contaminated site (Zayed and Terry, 2003). TF values can describe the movement and distribution of HMs into the plants. The Ni(II)-treated seedlings showed a TF above 0.61, whereas the Pb(II)-treated seedlings showed TF over to 0.17.
Our results indicate that P. laevigata is a suitable species for Pb (II) and Ni (II) removal. Furthermore the results found in this work indicate that P. laevigata can be considered a promising candidate for large scale HM phytoremediation purposes since not only Ni(II) and Pb(II) but also Cr(VI) and Cd(II) have been accumulated in high levels (Buendía-González et al., 2010). Interestingly, Buendía-González et al. (2010) obtained similar results regarding the effect of these metals (0.25-1.70 mM of Cr(VI) and 0.30-2.20 mM of Cd(II)) over germination, survival, uptake and accumulation P. laevigata responses. From these studies it may be inferred that P. laevigata resisted the toxic effects induced by Pb, Ni, Cr and Cd metals and exhibited good bioconcentration factors. P. laevigata is a species that grows in arid areas, thus it has a highly specialized physiology that makes it thrive in extreme environmental conditions. This suggests that P. laevigata possesses the genetic potential to respond at desert conditions and other types of stress, such as the presence of heavy metals in contaminated soils.
Conclusions
P. laevigata was able to germinate and grow in different Ni(II) and Pb(II) concentrations. The GR of the seedlings with both heavy metals was over 77 %. Also, the seedlings had the capacity to uptake Ni and Pb, but their ability to accumulate these heavy metals was different. The deleterious effect of heavy metals on seedlings growth was more pronounced when the medium was supplied with Ni(II) than with Pb(II). The Ni(II)-treated plants accumulated 2 430 and 3 895 mg of Ni kg-1 (d.b.) in root at 0.63 and 1.26 mM Ni(II), and translocated over to 61 % of the metal to the shoot tissues (1 925 and 2 582 mg Ni kg-1 (d.b.) at 0.63 and 1.26 mM Ni(II)), respectively. The roots of Pb(II)-treated seedlings accumulated 37 857 and 40 666 mg of Pb kg-1 (d.b.) at 1.5 and 3.0 mM Pb(II), and translocated over to 17 % of the metal to the shoot tissues (6 660 and 27 300 mg Pb kg-1 (d.b.) at 1.5 and 3.0 mM Pb(II)), respectively. This work showed that P. laevigata is an hyperaccumulator species of Pb(II) and Ni(II), and may be grown directly in soils highly contaminated with both HM, and thus it is a promising prospect for heavy metals phytoremediation purposes occurring in arid and semi-arid climates.
The results derived from these in vitro cultures could be used to predict the responses of plants to environmental contaminants, and to improve the design and thus to reduce the cost of subsequent conventional whole plant experiments.
Acknowledgements
The first author would like to thank the Consejo Nacional de Ciencia y Tecnologia (CONACyT) for her postdoctoral scholarship.
References
Akkenson, B. and Skerfing, S. (1985). Exposure in welding high nickel alloy. International Archives of Occupational and Environmental Health 56, 111-117. [ Links ]
Aldrich, M.V., Ellzey, J.T., Peralta-Videa, J.R., González, J.H. and Gardea-Torresdey, J.L. (2004). Lead uptake and the effects of EDTA on lead-tissue concentrations in the desert species mesquite (Prosopis spp.) International Journal of Phytoremediation 6, 195-207. [ Links ]
ATSDR, (2005). ToxFAQsTM for Nickel; Agency for Toxic Substances and Disease Registry, http://www.atsdr.cdc.gov/tfacts15.html [ Links ]
Baker, A.J.M. (1981). Accumulators and excluders-strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3, 643-654. [ Links ]
Baker, A.J.M. (1987). Metal tolerance. New Phytologist 106, 93-111. [ Links ]
Baker, A.J.M., McGrath, S.P., Reeves, R.D. and Smith, J.A.C. (2000) Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Phytoremediation of contaminated soil and water, (N. Terry and G. Bañuelos, eds.), Pp. 85107. Lewis Publishers, Boca Raton, FL. [ Links ]
Blaylock, M., Salt, D., Dushenkov, S., Zakharova, 0. , Gussman, C., Kapulnik, Y., Ensley, B. and Raskin, I. (1997). Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environmental Science and Technology 31, 860-865. [ Links ]
Buendía-González, L., Orozco-Villafuerte, J., Cruz-Sosa, F., Chávez-Ávila, V.M. and Vernon-Carter, E.J. (2007). Clonal propagation of mesquite tree (Prosopis laevigata Humb. & Bonpl. ex Willd. M.C. Johnston).I. Via cotyledonary nodes. In Vitro Cellular and Developmental Biology-Plant 43, 260-266. [ Links ]
Buendía-González, L., Orozco-Villafuerte, J., Cruz-Sosa, F., Barrera-Díaz, C.E. and Vernon-Carter, E.J. (2010). Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresource Technology, DOI:10.1016/j.biortech.2010.03.027. [ Links ]
Carrillo-Castañeda, G., Jufrez Munos, J., Peralta-Videa, J.R., Gomez, E., Duarte-Gardea, M., Tiemann, K.J. and Gardea-Torresdey, J.L. (2002). Alfalfa growth promotion by bacteria growth under iron limiting conditions. Advances in Environmental Research 6, 391-399. [ Links ]
Davies, J. (1986). Occupational asthma caused by nickel salts. Journal of the Society of Occupational Medicine 36, 29-31. [ Links ]
Doran, P.M. (2009). Application of plant tissue cultures in phytoremediation research: incentives and limitations. Biotechnology and Bioengineering 103, 60-76. [ Links ]
EPA (2009). Lead in paint, dust, and soil, U.S. Environmental Protection Agency. http://www.epa.gov/lead/ [ Links ]
Frankenberger, W.T. (2002). Preface. In: Environmental chemistry of arsenic (W.T. Frankenberger, ed.) Marcel Dekker, New York. [ Links ]
George, E.F. and de Klerk, G.J. (2008). The components of plant tissue culture media I: macro- and micro-nutrients. In: Plant propagation by tissue culture, (E.F. George, G.J.de Klerk, and M.A. Hall, eds.). Pp. 65-113. Springer, Dordrecht, The Netherlands. [ Links ]
Huang, J., Chen, J., Berti, W. and Cunningham, S. (1997). Phytoremediation of lead-contaminated soils: Role of synthetic chelates in lead phytoextraction. Environmental Science and Technology 31, 800-805. [ Links ]
Johnson, D. and Hale, B. (2004). White birch ( Betula papyrifera Marshall) foliar litter decomposition in relation to trace metal atmospheric inputs at metal-contaminated and uncontaminated sites near Sudbury, Ontario and Rouyn-Noranda, Quebec, Canada. Environmental Pollution 127, 65-72. [ Links ]
Jonak, C., Nakagami, H. and Hirt, H. (2004). Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiology 136, 3276-3283. [ Links ]
Kukier, U. and Chaney, R.L. (2004). In situ remediation of nickel phytotoxicity for different plant species. Journal of Plant Nutrition 27, 465-495. [ Links ]
Kumar, P.B.A.N., Dushenkov, V., Motto, H. and Raskin, I. (1995). Phytoextraction: the use of plants to remove heavy metals from soils. Environmental Science and Technology 29, 1232-1238. [ Links ]
Lupankwa, K., Love, D., Mapani, B.S. and Mseka, S. (2004). Impact of a base metal slimes dam on water systemms, Madziwa Mine, Zimbabwe. Physics and Chemistry of the Earth 29, 1145-1151. [ Links ]
Murashige, T. and Skoog, F. (1962). A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum 15, 473-497. [ Links ]
Niu, Z.X., Sun, L.N., Sun, T.H., Li, Y.S. and Wang, H. (2007). Evaluation of phytoextracting cadmium and lead by sunflower, ricinus, alfalfa and mustard in hydroponic culture. Journal of Environmental Science 19, 961-967. [ Links ]
Peer, W.A., Baxter, I.R., Richards, E.L., Freeman, J.L. and Murphy, A.S. (2005). Phytoremediation and hyperaccumulator plants. In: Topics in current genetics, Vol. 14: Molecular biology of metal homeostasis and detoxification, (M.J. Támas and E. Martinoia, eds.), Pp. 299-340. Springer-Verlag, Berlin. [ Links ]
Peralta, J.R., Gardea-Torresdey, J.L., Tiemann, K.J., Gomez, E., Arteaga, S., Rascon, E. and Parsons, J.G. (2001). Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L). Bulletin of Environmental Contamination and Toxicology 66, 727-734. [ Links ]
Peralta-Videa, J.R., de la Rosa, G., González, J.H. and Gardea-Torresdey, J.L. (2004). Effects of the growth stage on the heavy metal tolerance of alfalfa plants. Advances in Environmental Research 8, 679-685. [ Links ]
Pilon-Smits, E. (2005). Phytoremediation. Annual Review of Plant Biology 56, 15-39. [ Links ]
Reeves, R.D. and Brooks, R.R. (1983). Hyperaccumulation of lead and zinc by two metallo-phytes from mining areas of Central Europe. Environmental Pollution - Series A 31, 277-285. [ Links ]
Saier, M.H. Jr. and Trevors, J.T. (2008). Phytoremediation. Water, Air, and Soil Pollution DOI 10.1007/s11270-008-9673-4. [ Links ]
Salvatore, M.D., Carafa, A.M. and Carratu, G. (2008). Assessment of heavy metals phytotoxicity using seed germination and root elongation test: A comparison of two growth substrates. Chemosphere 73, 1461-1464. [ Links ]
Wu, J., Hsu, F. and Cunningham, S. (1999). Chelate-assisted Pb phytoextraction: Pb availability, uptake, and translocation constraints. Environmental Science and Technology 33, 1898-1904. [ Links ]
Xintaras, C. (1992). Analyses paper: Impact of lead-contaminated soil on public health. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry http://www.atsdr.cdc.gov/cxlead.html [ Links ]
Zayed, A.D. and Terry, N. (2003). Chromium in the environment: factors affecting biological remediation. Plant and Soil 249, 139-156. [ Links ]
Zhao, F.J., Lombi, E. and McGrath, S.P. (2003). Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil 249, 37-43. [ Links ]














