INTRODUCTION
Hepatocellular carcinoma (HCC) is a highly prevalent and lethal cancer type being the third leading cause of cancer-related deaths worldwide. Etiologically, HCC usually develops in chronic hepatitis B virus (HBV) carriers, especially in East Asia and sub-Saharan Africa, where HBV is endemic. For these HCC patients, curative tumor resection remains the most effective treatment and the prognosis of HCC was greatly improved. However, high opportunity of intrahepatic recurrence remains a major obstruction for further improving the prognosis of HCC patients after curative resection. It has been hypothesized that early and late intrahepatic recurrence of HCC was attributable to two different mechanisms: intrahepatic metastasis and de novo multicentric carcinogenicity, respectively. Imamura, et al. proposed a convenient framework to clinically differentiate each type of recurrence as ‘early’ or Tate’ based on a cut-off of 2 years postoperatively. Potentially, risk factors including tumor size, nodule number, positive surgical margin, microvascular invasion, tumor node metastasis (TNM) stage, and Edmondson’s grade were significantly related to early HCC recurrence. With successful risk surveillance of HCC development and curative resection, the development of early recurrence can be avoided in a higher number of patients, thereby helping them survive longer. Meanwhile, the risk factors contributing to late recurrence after surgery have not been investigated on a comprehensive basis. Although the precise mechanism underlying recurrent carcinogenesis associated with HBV in the remaining liver in patients who have undergone curative therapy remains unclear, it is possible that viral replication and integration of sub-genomic HBV DNA fragments into the host liver cells play a key role in contributing to the carcinogenic process. According to current opinions, there is sufficient and powerful evidence connecting elevated serum HBV DNA levels with an increased risk of HCC recurrence and death after curative resection. In particular, patients with serum HBV DNA levels > 2,000 IU/mL at study entry have an increased risk of HCC recurrence. In contrast, those with HBV DNA levels < 2,000 IU/mL are usually designated inactive or low-risk HBV carriers. However, even these low viral load patients still carry a high incidence of HCC recurrence. Therefore, it is necessary to identify factors other than viral load that are predictive of HCC in these low-risk patients. Recently, the quantification of hepatitis B surface antigen (HBsAg) level has been introduced as a surrogate marker for the management of chronic hepatitis B infection. A Taiwanese cohort study demonstrated that high HBsAg levels predicted the risk of HCC in hepatitis B e antigen (HBeAg) negative patients with low HBV DNA levels. Tseng, et al. also demonstrated that high HBsAg levels could predict disease progression in HBeAg negative patients. However, there are only a few studies assessing the role of HBsAg levels as a risk factor of recurrence in HCC. Thus, the effect of HBsAg levels on HCC recurrence after curative resection in low viral load patients remains uncertain.
We therefore aimed to conduct a retrospective cohort study to assess the impact of serum HBsAg levels on the prognosis of HCC among patients with low viral load (< 2,000 IU/mL). In addition, based on the hypothesis that early and late intrahepatic recurrence of HCC was attributable to different mechanisms, we also investigated the risk factors for early (≤ 2 years) and late (> 2 years) intrahepatic recurrence, separately.
MATERIAL AND METHODS
Patients
Between January 2009 and December 2011, 192 patients with HBV-related HCC who underwent curative resection as the primary therapy at the Affiliated Hospital of Nantong University were enrolled in this retrospective cohort study based on the following inclusion criteria:
Positive serum HBsAg for at least 12 months with a serum HBV DNA level < 2,000 IU/mL.
Complete resection of all tumor nodules and a surgical free margin of more than 5 mm by pathological examination.
No cancerous thrombus in the portal vein (main trunk or two major branches), hepatic veins, or bile duct.
No more than three tumor nodules.
No extrahepatic metastasis.
No adjuvant antiviral therapy with nucleoside analogue was given before or after the operation.
Death in the hospital not due to postoperative hepatic failure; and
Presence of early recurrence at least 3 months postoperatively (excluding pre-existing metastases before HCC resection).
The histological grade proposed by Edmondson and Steiner, maximal tumor size, nodule number, capsular formation around the tumor, microvascular invasion, liver cirrhosis, and extent of resection were also determined. The protocol was approved by the Ethics Committee of the Affiliated Hospital of Nantong University in accordance with the tenets of the Declaration of Helsinki.
Follow-up and treatment for tumor recurrences
After surgical resection of HCC, all 192 patients were assessed every 3-6 months with dynamic contrast-enhanced computer tomography (CT) and/or magnetic resonance imaging (MRI), as well as with measurement of a serum tumor marker such as AFP. Hepatic angiography was performed when recurrence was suspected. Postoperatively, 19 patients received interferon-alpha (IFN-a) treatment, which was started at a pilot dose of 3 million units (MU) two times a week by intramuscular injection for 2 weeks, then 5 MU three times a week for 18 months. The IFN-a treatment was terminated when recurrence was confirmed. Diagnosis of intrahepatic recurrence was based on histopathologic examination of tumor tissue in 23 patients undergoing repeat hepatic resection and on the characteristic appearance of CT, MRI, and hepatic angiography in 85 patients. When recurrence was confirmed, the patients received the optimal treatment modality for each case based on the number and location of the recurrent tumors and liver function tests. Treatment of recurrent HCC included repeat hepatic resection, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), chemotherapy or radiotherapy, and transcatheter arterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy.
Evaluation of prognostic clinical and pathological variables
Clinical and pathological factors in this study were selected for their potential relationship to prognosis on the basis of previous studies, and included age (continuous variable), gender (male or female), alanine aminotrans ferase level (continuous variable), total bilirubin level (continuous variable), albumin level (continuous variable), prothrombin time (continuous variable), HBeAg status (positive or negative), serum HBsAg level (≤ 250 or > 250 IU/mL), co-existing hepatitis C virus (HCV) infection (yes or no), liver cirrhosis (yes or no), Child-Pugh grade (A or B), Okuda stage (I or II), serum AFP level (≤ 400 or > 400 ng/mL), tumor size (≤ 3 or > 3 cm in diameter), number of tumor nodules (single or multiple), tumor capsular formation (yes or no), microvascular invasión (yes or no), differentiation of tumor cells (Edmondson’s classification I/II or III/IV), postoperative IFN-α therapy (yes or no), and extent of resection (minor, < 3 segments or major, ≥ 3 segments) based on Couinaud’s nomenclature. Serum HBV DNA levels were determined using the fluorescein quantitative polymerase chain reaction (FQ-PCR) detection system (TaqMan; Roche Diagnostics, Branchburg, NJ, USA). HBsAg level was quantified by the automated chemiluminescent microparticle immunoassay (Architect HBsAg, Abbott, IL, USA). The detection range of the Architect HBsAg assay was 0.05 to 250 IU/mL.
Statistical analysis
Data were presented as means ± SD, proportions, or median (range). Clinical and pathological data at the time of resection were analyzed to identify factors that influenced prognosis via the Cox proportional hazards model. The overall survival rate was calculated from the date of resection to the date of death or last follow-up. The cumulative recurrence rates were calculated from the date of resection to the date when tumor recurrence was diagnosed, or if recurrence was not diagnosed at the time of study, cases were censored on the date of death or last follow-up. To investigate factors contributing to early and late recurrence separately, we set 2 years as the cut-off between early and late recurrence, as suggested by Imamura’s study. Survival curves were calculated by the Kaplan-Meier method and differences were compared by the log-rank test. Multivariate analysis was performed by the Cox proportional hazards regression model. Statistical significance was defined by a P value < 0.05. Statistical analyses were performed using the Statistical Program for Social Sciences (SPSS v.11.5 for Windows; SPSS, Inc., Chicago, IL, USA).
RESULTS
During a median observation period of 38.5 months (with a range of 3 to 72 months), recurrence was found in 108 patients (56.3%). Of these, 23 patients received a second resection, 7 patients received RFA, 56 patients received TACE, 14 patients received chemotherapy or radiotherapy or PEI, and the remainder received a conservative treatment. During follow-up, 91 patients died, 14 of whom did not have recurrent HCC but died due to other causes, mainly chronic deterioration of liver function and massive upper digestive tract bleeding caused by esophageal varices. The overall survival rates at 1-, 3-, and 5- year after curative resection were 94.2%, 64.0%, and 45.2%, respectively. The cumulative recurrence rates at 1-, 3-, and 5-year after curative resection were 22.4%, 46.5%, and 67.0%, respectively. The baseline demographic, clinical, and pathological features of the study population are depicted in table 1.
Factors associated with overall survival in univariate and multivariate analyses
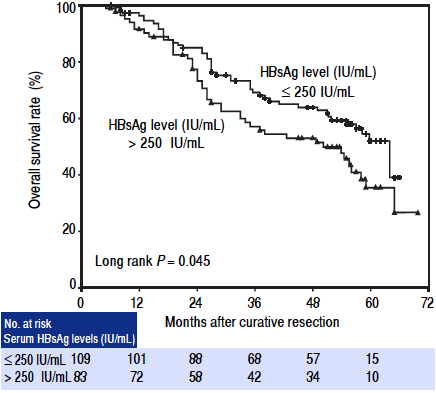
In the univariate analysis, serum HBsAg level > 250 IU/mL (hazards ratio (HR): 1.517, 95% confidence interval (Cl): 1.005 - 2.292, P = 0.047), tumor size > 3 cm in diameter (HR: 1.673, 95% Cl: 1.086 - 2.577, P = 0.020), and presence of microvascular invasion (HR: 1.751, 95% Cl: 1.115 - 2.750, P = 0.015) were significantly associated with poor overall survival postoperatively. Other clinical factors including sex, age, co-existing HCV infection, serum albumin level, total bilirubin level, aminotransferase level, prothrombin time, HBeAg status, presence of cirrhosis, tumor number, Child-Pugh grade, Okuda stage, extent of resection, and postoperative IFN-a treatment were not associated with survival (Table 2). The 1-, 3-, and 5-year overall survival rates for high HBsAg level patients (> 250 IU/mL) were 91.4%, 57.1%, and 35.4%, respectively, in contrast to 96.3%, 69.1%, and 52.0%, respectively, for low HBsAg level patients (≤ 250 IU/mL). Patients with high serum HBsAg levels had significantly lower survival rates than those with low HBsAg levels (log rank P = 0.045; Figure 1). However, in the multivariate analysis, the presence of microvascular invasion (HR: 1.740, 95% Cl: 1.108 - 2.734, P = 0.016) was the only independent risk factor for overall survival (Table 2).
Table 2 Factors identified on univariate and multivariate Cox regression analyses that influenced overall survival in HCC patients undergoing curative resection.
Factors associated with early and late recurrence after curative resection
During follow-up, 108 patients had HCC recurrence. The median time from tumor resection to the detection of recurrence was 24.5 months (with a range of 3 to 66 months). Factors related to early recurrence (≤ 2 years) and late recurrence (> 2 years) were investigated separately, as suggested by Imamura’s study4 (Tables 3 and 4).
Table 3 Factors identified on univariate and multivariate Cox regression analyses that influenced early recurrence (≤ 2 years) in HCC patients undergoing curative resection.
Table 4 Factors identified on univariate and multivariate Cox regression analyses that influenced late recurrence (> 2 years) in HCC patients undergoing curative resection.
Among 192 patients, 74 had recurrence within 2 years. Stratified Cox regression analysis identified four variables as factors related to early recurrence: AFP > 400 ng/mL (HR: 2.014, 95% Cl: 1.223 - 3.316, P = 0.006), tumor size > 3 cm in diameter (HR: 2.285, 95% Cl: 1.377-3.791, P = 0.001), presence of microvascular invasion (HR: 2.546, 95% Cl: 1.595 - 4.064, P < 0.001), and presence of capsular formation (HR: 0.620, 95% Cl: 0.392-0.979, P = 0.040). The presence of high HBsAg levels was not associated with a significantly higher cumulative risk of tumor recurrence during first 2 years after curative resection (log rank P = 0.227). In the multivariate analysis, AFP > 400 ng/mL (HR: 1.679, 95% Cl: 1.007 - 2.801, P = 0.047), tumor size > 3 cm in diameter (HR: 1.871, 95% Cl: 1.103-3.175, P = 0.020), and presence of microvascular invasion (HR: 1.917, 95% Cl: 1.163-3.159, P = 0.011) were independently associated with early tumor recurrence.
Factors related to late recurrence were investigated in 96 patients who were recurrence-free during the first 2 years. Recurrence was detected in 34 patients during follow-up. Univariate analysis identified three risk factors contributing to late recurrence: the presence of cirrhosis (HR: 2.821, 95% Cl: 1.089 - 7.310, P = 0.033), multiple tumor number (HR: 2.504, 95% Cl: 1.206 - 5.200, P = 0.014), and serum HBsAg > 250 IU/mL (HR: 2.155, 95% Cl: 1.094 - 4.248, P = 0.026). Patients with high HBsAg levels at resection had a significantly higher cumulative late recurrence rate than those with low HBsAg levels (log rank P = 0.022; Figure 2). In the multivariate analysis, the presence of multiple tumor nodules (HR: 2.446, 95% Cl: 1.172-5.104, P = 0.017) and serum HBsAg > 250 IU/mL (HR: 2.109, 95% Cl: 1.068 - 4.165, P = 0.032) were independent risk factors associated with late recurrence.
DISCUSSION
In this retrospective cohort study, we focused primarily on the correlation between serum HBsAg levels and prognosis of HBV-related HCC patients with low viral load (< 2,000 IU/L) after curative resection. Multivariate analysis demonstrated that early recurrence was independently associated with the presence of microvascular invasion, AFP > 400 ng/mL, and tumor size > 3 cm in diameter. On the other hand, the risk factors for late recurrence were high HBsAg levels (> 250 IU/mL) and the presence of multiple tumor number. However, the presence of microvascular invasion was the only independent risk factor related to overall survival in low viral load subjects after curative resection.
Previous studies have confirmed that high AFP level, tumor size, multiple nodule number, vascular invasion, a positive surgical margin, and Edmondson’s grade are prognostic factors predicting tumor recurrence after curative resection. Early recurrence, which appears with 2 years postoperatively, is associated with tumor-related factors; late recurrence appears > 2 years postoperatively, and is considered to be associated with hepatic inflammation and viral replication. The latter is clonally independent from the primary tumor. However, there has been a paucity of studies concerning the HBV status of patients undergoing HCC resection in the past, probably due in part to the lack of interest in hepatitis virology among liver surgeons who manage these patients. Till date, several studies assessing the effect of HBV status on the prognosis of HCC patients receiving curative resection have been reported. Some potential viral risk factors associated with prognosis have been identified, such as seropositivity of HBeAg, high viral load, genotype, and specific viral sequence mutations. Among these factors, an elevated serum HBV DNA level is widely considered to be the most important risk factor for the poor prognosis of HCC patients after tumor resection, as well as for cases most suitable for intervention. In some case series studies on the recurrence of HBV-related HCC after curative resection, patients with a high viral load at study entry had a significantly higher risk of tumor recurrence than those with a low viral load. In our previous study, during a mean follow-up period of 33.7 months after curative resection, we found that serum HBV DNA levels of 104 copies/mL or more at pre-operation was an independent risk factor for HCC recurrence. However, even in these low viral load patients who received tumor resection, the risk of tumor recurrence 2 years postoperatively remained. Recently, the introduction of HBsAg quantification has attracted much attention for its value in stratifying the risk of disease progression and predicting the prognosis of patients with chronic HBV infection. A Taiwanese cohort study demonstrated that high HBsAg levels predicted the risk of HCC in HBeAg-negative patients with low HBV DNA levels.16 However, the role of HBsAg in the recurrence of HBV-related HCC in low HBV DNA level subjects was seldom reported yet. In this study, we evaluated the role of serum HBsAg levels on the prognosis of low viral load HCC patients (< 2,000 IU/mL). Our results revealed that in a subgroup of 96 patients who were recurrence-free for first 2 years, the presence of multiple tumor nodules and HBsAg > 250 IU/mL were independent risk factors for late recurrence. However, high HBsAg levels were not associated with early tumor recurrence or overall survival in the multivariate analysis. In a recent study, Huang, et al. also confirmed that a preoperative serum HBsAg ≥ 1,000 IU/mL was an independent risk factor for HCC recurrence in patients with low HBV DNA levels. In our latest meta-analysis, despite recommended cutoffs and significant heterogeneity among the different included studies, the results still supported the opinion that the presence of high HBsAg level was associated with a high risk of late recurrence after curative resection of HCC, although the rate of early recurrence was not higher in high HBsAg patients compared to the patients with low HBsAg level.
The precise mechanism for recurrent carcinogenesis associated with high HBsAg levels in patients with chronic HBV infection remains unclear. As we known, HBsAg is the hallmark of HBV infection and was first reported by Blumberg, etal. in 1968. Measurement of HBsAg is usually used to identify HBV infection. The level of serum HBsAg reflects the transcriptional activity of covalently closed circular DNA (cccDNA). It is generally believed that serum HBsAg concentration is significantly correlated with intrahepatic amounts of total HBV DNA and cccDNA. HBsAg is mainly derived from the integrated form of HBV DNA rather than the episomal form, and patients with a low viral load who have high HBsAg levels may have more hepatocytes with HBV integration than those who have low HBsAg levels. It has been speculated that the higher risk of HCC in high HBsAg level patients might be attributed to the increased genomic instability as a result of integrated viral sequences, which plays an important role in hepatocarcinogenesis. Additionally, in theory, late recurrence (> 2 years) has been speculated to be associated with the background liver disease condition, such as hepatic inflammation and liver damage. High HBsAg levels in patients may indicate higher cccDNA levels and confer further increased viral replication, leading to even higher HCC risk. When these lines of evidence are taken together, these studies may provide a new insight into the role of serum HBsAg levels in the recurrence of HBV-related HCC after curative resection. Based on our data, preoperative measurement of HBsAg levels in addition to HBV DNA levels could be used as a potential clinical marker useful for the prediction of prognosis in HBV-related HCC.
Other independent risk factors associated with HCC prognosis after curative resection that were identified in this study included preoperative AFP ≥ 400 ng/mL, multiple tumor number, and the presence of microvascular invasion. These findings were consistent with those described previously. Patients with high AFP level tended to have greater tumor size, bilobar involvement, massive or diffuse types, and tumor vascular invasion. The presence of microvascular invasion was consistently reported as strongly predictive of intrahepatic metastasis. Meanwhile, tumor multiplicity was thought to be a variable reflecting increased carcinogenicity of the background liver tissue. Our stratified analysis demonstrated that the presence of multiple tumor nodules was an independent risk factor for late recurrence. This finding strongly supported the hypothesis that late recurrence was mainly attributable to de novo multicentric carcinogenicity.
There is strong evidence linking elevation in serum HBV DNA levels and HCC recurrence after tumor resection in chronic hepatitis B. In the present study, we further showed that high HBsAg levels (> 250 IU/mL) were associated with a significantly higher later recurrence rate after curative resection in low viral load patients. There are also several limitations in this study. First, HBsAg quantification was based on a single blood sample obtained at surgery; therefore, we could not assess the risk of fluctuation in serum HBsAg levels during follow-up on the prognosis of HCC. Second, as a retrospective cohort study, it is difficult to reach a firm conclusion and a large- scale, well-designed prospective study with long-term follow-up should be conducted to solve this issue in the future.
In summary, we found that the independent risk factors for early recurrence after curative resection of HBV-related HCC were the presence of microvascular invasion, AFP > 400 ng/mL, and tumor size > 3 cm in diameter. Meanwhile, late recurrence was associated with high preoperative HBsAg levels and multiple tumor number at resection in low viral load HCC subjects. If the role of HBsAg levels in predicting HBV-related HCC prognosis after curative resection is further confirmed, this new potential biomarker could be incorporated into the risk calculator for HCC recurrence, particularly in patients with low viral load.











 nueva página del texto (beta)
nueva página del texto (beta)