INTRODUCTION
Protease inhibitors (PIs) have improved sustained virological response (SVR) in patients with chronic hepati tis C virus (HCV) when used in combination with peg-interferon and ribavirin (PR).1 Hematological adverse events with PR, like interferon (IFN)-related central myelosuppression or ribavirin (RBV)-related hemolytic anemia are usually mild.2 Few cases of hematological serious adverse events (SAEs) in HCV infected patients receiving antiviral treatment with PR have been reported in the literature: two cases of severe aplastic anemia related to IFN-alfa and two cases of pure red cell anemia (PRCA) associated with peg-IFN and RBV, respectively.3 , 4
Addition of the new PIs to PR has shown a high frequency of mild hematological toxicity.5 , 6 Our group has recently described severe pancytopenia or aplastic anemia in seven patients during triple therapy with telaprevir (TVR).7 Of note, these hematological complications resulted in a high mortality rate (three out of seven patients died). Interestingly, only one of these seven patients had both risk factors (low levels of platelet < 100 x 109/L and albumin < 35 g/L) for developing adverse events, previously described in cirrhotic patients of the CUPIC cohort.8
We here report two cases of severe agranulocytosis/ aplastic anemia using boceprevir (BOC) or simeprevir (SMV) in IFN-based combination and 2 additional cases of severe myelosupression with IFN-free therapy with sofosbuvir and simeprevir (SMV) plus RBV (Table1 summarises main clinical and laboratory features).
Table 1 Main clinical and laboratory features at baseline and during follow-up.
INR: International normalized ratio. CP: Child Pugh score. MELD: score Model for End-Stage Liver Disease. Wk: week. Wk t at toxicity: weeks of treatment at toxicity development. AA: Aplastic anemia. AG: agranulocytosis. Non-Hem An: non hemolytic anemia. BMB: bone marrow biopsy. BMA: bone marrow aspiration. EPO: Erythropoietin. RBC: red blood cell units of transfusion. G-CSF: Granulocyte colony stimulating factor. Platelets (units): units of transfusion. SVR 12: sustained viral response at week 12 of treatment. SVR 24: sustained viral response at week 24 of treatment.
CASE STUDIES
Case 1
This patient was a 42 year-old man with HCV-related cirrhosis (genotype 1a/1b IL28CC), without esophageal varices. His baseline blood tests were normal with the exception of thrombocytopenia (114 x 109/L). He started lead-in with PR and at week 4, BOC was added. RBV dose was decreased and erythropoietin (EPO) was started due to hemoglobin (Hb) reduction (103 g/L). At week 8, he had undetectable viral load (VL) and neutrophil count showed grade 1 neutropenia (1800 cells/μL). At week 12, he was admitted to hospital because of fever and pancyto penia (neutrophils of 0 cells/μL, Hb of 81 g/L and platelets of 39 x 109/L). Antiviral treatment was withdrawn and antibiotics were initiated. A bone marrow aspiration showed agranulocytosis (Figure 1). He required granulocyte colony stimulating factor (G-CSF) for 5 days, EPO and platelet transfusions (Figure 2). Five days after admission, his neutrophil count recovered and, at last follow-up, the patient has a normal blood cell count.
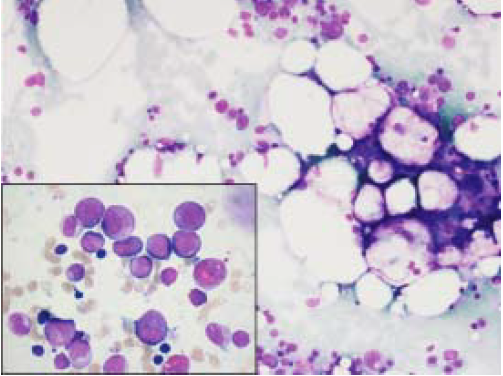
Figure 1 Bone marrow aspiration showing moderate hypocellularity and fat replacement. H&E, 200x. In the inset, promyelocytes and myelocytes consistent with early granulocytic recovery in bone marrow. H&E, 1000x.
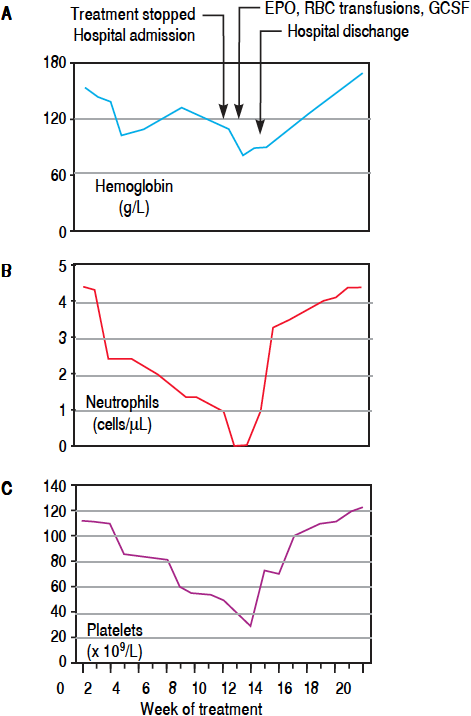
Figure 2 Hemoglobin (A), neutrophils (B) and platelets (C) profiles over a 20 weeks period from starting oral anti-hepatitis C triple therapy in case 1 with highlighted relevant clinical events. EPO: Erythropoietin. RBC: red blood cell units of transfusion. G-CSF: granulocyte colony stimulating factor.
Case 2
This patient was a 64-year-old man without comorbidities and HCV infection (genotype 1b ILB28CT) with advanced fibrosis (10.4 kilopascals) on transient elastography (TE). Baseline tests were normal except for thrombocytopenia (133 x 109/L). At week 4 after starting PR plus SMV, VL was undetectable. Hemoglobin levels dropped from 164 to 115 g/L, thus EPO alpha was started. At week 8, neutropenia appeared (620 cells/μL) and G-CSF was given. At week 12, blood tests showed severe pancytopenia with Hb of 75 g/L, neutrophils of 0 cells/μL and platelet count of 15 x109/L. HCV treatment was discontinued and red blood cells and platelet transfusions were initiated. The patient developed fever and was admitted to the hospital. Intensive fluid therapy and broad-spectrum antibiotic was started. A bone marrow aspiration showed severe hypocellularity and a bone marrow biopsy confirmed the diagnosis of aplastic anemia. An extensive work-up was initiated and other causes of acquired bone marrow failure such us myelodysplastic syndrome, leukemia, megaloblastic anemia, paroxysmal nocturnal hemoglobinuria and viral hemophagocytic syndrome were excluded. Then, it is reasonable to associate antiviral treatment as the most plausible cause of hematological toxicity in this case. Treatment with cyclosporine and prednisone was started at 21 days from admission. Though still pancytopenic, he was discharged after 49 days of hospitalization. At last follow-up, 8 months after discontinuing HCV treatment, he is still receiving immunosuppressive therapy, EPO and transfusions. Fortunately, VL remains undetectable.
Case 3
This patient was a 53-year-old woman with genotype 1a HCV-related cirrhosis with esophageal varices and cryoglobulinemia. She had previously received a first course of PR developing severe anemia. Five years later, the patient started RBV, SOF and SMV. At week 4, she achieved undetectable VL but anemia (Hb of 72 g/L) was found, requiring RBV dose reduction, red blood cell transfusion and EPO. At week 6, anemia worsened (Hb of 55 g/L) while neutrophils and platelets remained normal. Laboratory tests excluded hemolysis and vitamin deficiency. RBV and SMV were withdrawn, and daclatasvir (DCV) was added to SOF. A bone marrow examination performed 5 days later, showed incipient recovery of erythroid precursor cells in bone marrow. Three weeks later, Hb levels returned to normal. She could finish 16 weeks of treatment maintaining undetectable VL and normal blood counts.
Case 4
This patient was a 58-year-old man with genotype 1b HCV cirrhosis associated to type 2 cryoglobulinemia with lymphoplasmacytic lymphoma. The patient had received rituximab for his lymphoma twice, both times reaching remission. He received a first course of PR without showing viral response. Eight years later, treatment with SMV and SOF plus RBV was started. At week 4, he achieved undetectable VL but developed grade 3 anemia. Dose of RBV was decreased and red blood cell transfusions and EPO were administrated. At week 6, the patient presented with neutropenic fever (neutrophils of 600 cells/μL) and severe anemia (Hb of 66 g/L). Broad-spectrum antibiotics plus G-CSF were started and RBV was stopped. Bone marrow aspiration ruled out recurrence of the lymphoproliferative disorder. White cells counts recovered in a week. At week 12, anti-HCV therapy was completed and anemia fully recovered at week 15. Unfortunately, at week 23, the patient developed refractory status epilepticus secondary to an encephalitis by BK virus and died.
DISCUSSION
In the last few years, new antiviral agents have been approved for the treatment of HCV, including first and second generation PIs. Several PIs-based regimens have improved antiviral efficacy but can cause severe adverse events (AEs) including bacterial infections and clinical decompensation of liver disease.9 In addition, severe pancytopenia and aplastic anemia during triple therapy, including TVR, have been recently reported.7 This severe toxicity has shown a high risk of mortality, being imperative the close monitoring of these patients. Recently, second generation PIs (SMV) have shown a better safety profile and lower risk of developing severe anemia than TVR, a first generation PI.10
IFN, in addition to its well-known antiviral effect, exerts antiproliferative activity on many cell types, including hematopoietic cells.4 This property may lead to cytopenias that can interfere with the successful clinical application of IFN.
In contrast, RBV is a cytotoxic agent and its accumulation in erythrocytes produces oxidative membrane damage, leading to an accelerated extravascular hemolysis by the reticulo-endothelial system.11 At a low dose, it decreases half-life of red cells with a reversible effect when the drug is discontinued, and at high doses, RBV also inhibits the release of red cells from the bone marrow.12 However, only few cases of severe hematologic toxicity induced by IFN/RBV therapy have been reported in HCV patients after many years of use in the daily practice.13 , 14
We here report 4 cases of severe hematological toxicity in HCV patients receiving first or second generation PIs with or without IFN, but always with RBV. Interestingly, two of these cases receiving IFN-containing combination were also under BOC or SMV, a first and a second generation PI, respectively.
Causes of hematological toxicity like drug toxicity (EPO, antibiotics, anti-inflammatory drugs, anticonvulsants), vitamin deficiency, autoimmune reactions, hemolysis, bone marrow infiltration or malignancy were ruled out by specific diagnostic test or absence of temporal relationship. EPO-related anemia could also be discarded since all our patients fully recovered their red cell counts while maintaining EPO administration, and in addition, to the best of our knowledge, no other cytopenias have been reported with this agent.
Of note, all patients had advanced fibrosis or cirrhosis, but none of them was decompensated and only one of the patients treated without IFN had platelets < 100 x 109/L and albumin < 35 g/L at the beginning of treatment.
In this study, we report 2 patients under triple therapy with PR and PIs who developed aplastic anemia or agranulocytosis, two life-threatening hematologic complications. Interestingly, one patient was receiving BOC, first generation PIs and the other one SMV, a second generation PI. Although the association with PIs cannot be totally proved, our observations suggest a sort of class-effect of PIs in the development of severe hematological toxicity. However, an alternative explanation could be an additive toxic effect of PIs to the well-known IFN/RBV hematological toxicity or an interaction of the drug combination in susceptible patients.
It is known that genetic variants leading to inosine triphosphatase (ITPA) deficiency protect against hemolytic anemia in HCV-infected patients receiving RBV,15 but the mechanism sustaining severe anemia during PIs based therapy is still unknown. Recent reports investigating the molecular mechanisms of anemia in anti-HCV triple therapy have shown that TVR-S isomer concentration is related to the concentration of RBV in plasma.16 It is supposed that TVR can produce a boosting effect on plasma RBV and its intra-erythrocytic concentration, finally leading to a toxic effect. So that, it has been suggested a bimodal pattern: an early phase mainly due to acquired spherocyticlike hemolytic anemia and a late phase showing hyporegenerative features, most likely related to the combined effects of PR and PIs on erythropoiesis.17
In addition, we have also reported two additional patients who developed severe anemia and one of them also grade 4 neutropenia under IFN-free regimens (SMV and SOF plus RBV). Although this last patient had received rituximab for lymphoma treatment, at the time of starting HCV treatment he was in complete remission of the lymphoma and no clinical or laboratory findings due to cryoglobulinemia were evident. In addition, the coexistence of anemia and neutropenia is not consistent with the clinical picture seen in cases with delayed neutropenia induced by rituximab. To our knowledge, these are the first reported cases of severe hematologic toxicity associated to this three-agent regimen. One patient developed PRCA after 4 weeks of treatment, and the other initiated severe bicytopenia at 6 weeks of starting therapy. RBV was held in both patients, but SMV and SOF were maintained in one, and SMV was changed to DCV in the other. Although both patients required red blood cell transfusions and EPO, anemia rapidly improved in the following weeks.
This prompt recovery of anemia after RBV withdrawal in our patients suggests that RBV probably is the main causative agent of anemia in IFN-free regimens. However, given the low incidence of PRCA due to RBV in the literature and the resolution of anemia after changing SMV for DCV in our case 4, we cannot completely rule out a possible additive toxic effect of SMV. Considering the high antiviral activity of SMV and SOF, we suggest that RBV might be avoided with these new regimens, especially in those patients with advanced liver disease and in those with mild cytopenias previous starting HCV treatment.18 However, further studies are needed to evaluate the role of RBV in these new IFN-free regimens.
These cases of severe and life-threatening adverse events have occurred in our center in a period of scarcely 2 years in which about 170 patients received treatment with PIs. This observation highlights an unexpected high incidence of hematologic toxicity, something not previously observed by our group despite decades of treatment with PR. Moreover, these hematologic complications entail large amounts of health resources, including the use of support treatment with G-CSF and EPO, transfusions, broad spectrum antibiotics, antifungals and even longterm hospitalization. In order to optimize resource use, we recommend a close cooperation between hematology and hepatology teams,19 as we previously suggested.20
In conclusion, severe hematological adverse events in patients treated with PIs and RBV are more frequent than expected, an observation suggesting either a possible class effect of PIs in the development of these toxicities or an interaction to the drug combination in susceptible patients. Given the life-threatening character of these complications in some patients, we highly recommend to promptly discontinue RBV if blood cells significantly drop and to avoid it in cases with increased risk of development of hematological toxicity. In addition, close cooperation between hematologists and hepatologists is also advisable.
ABBREVIATIONS
BOC: |
boceprevir. |
DCV: |
daclatasvir. |
EPO: |
erythropoietin. |
G-CSF: |
granulocyte colony stimulating factor. |
Hb: |
hemoglobin. |
HCV: |
chronic hepatitis C virus. |
IFN: |
peg-interferon. |
ITPA: |
inosine triphosphatase. |
Pis: |
protease inhibitors. |
PR: |
peg-interferon and ribavirin. |
PRCA: |
pure red cell anemia. |
RBV: |
ribavirin. |
SAEs: |
serious adverse events. |
SMV: |
simeprevir. |
SVR: |
sustained virological response. |
TE: |
transient elastography. |
TVR: |
telaprevir. |
VL: |
viral load. |











 nueva página del texto (beta)
nueva página del texto (beta)


