CASE PRESENTATION
Case 1
A 49 year old obese male presented as a referral to our institution in April 2014 for follow up of his HCV infection. His past medical history included chronic hepatitis C infection since 1995 (treated one year of interferon), hypertension and diabetes mellitus type 2. He was a former heavy drinker, but quit in 1995. He had a history of smoking (40 pack-years), prior recreational drug use (cocaine and marijuana) but no family history of liver disease. He was asymptomatic and his physical exam was unremarkable. Laboratory findings included: white blood cell count 9,000/μL, hemoglobin 16.6 g/dL, Platelets count 23.9 x 104/μL, total bilirubin 0.6 mg/dL, albumin 3.5 g/dL, aspartate aminotransferase 40 IU/L, alanine aminotransferase 46 IU/L, alkaline phosphatase 94 IU/L, and alpha-fetoprotein was 2.6 ng/mL. He previously had a liver biopsy in 1998 which showed mild inflammatory activity and portal fibrosis. He had a CT abdomen/pelvis with contrast in 2010 showing an 8 mm hypodense lesion in the right hepatic lobe. In 2013, a liver ultrasound showed grossly normal size and echotexture and no focal lesion.
On CT liver 3 phase protocol, the lesion in hepatic segment 7, measuring 1.6 x 1.3 cm demonstrated peripheral enhancement on the arterial and portal venous phases with the peripheral areas demonstrating wash-out on the delayed phase (Figure 1A). CT guided needle core biopsy revealed a well-differentiated hepatocellular carcinoma featuring an increase in hepatocyte plate thickness, but low nuclear/cytoplasmic ratio. Majority of neoplastic cells showed abundant foamy and pale cytoplasm (Figure 1B). There was a loss of reticulin framework by reticulin stain (Figure 1C left) and diffusely sinusoidal staining pattern of CD34 immunostain (Figure 1C right). Glypican-3 immunostain was essentially negative except minimal membrane staining (Figure 1D).
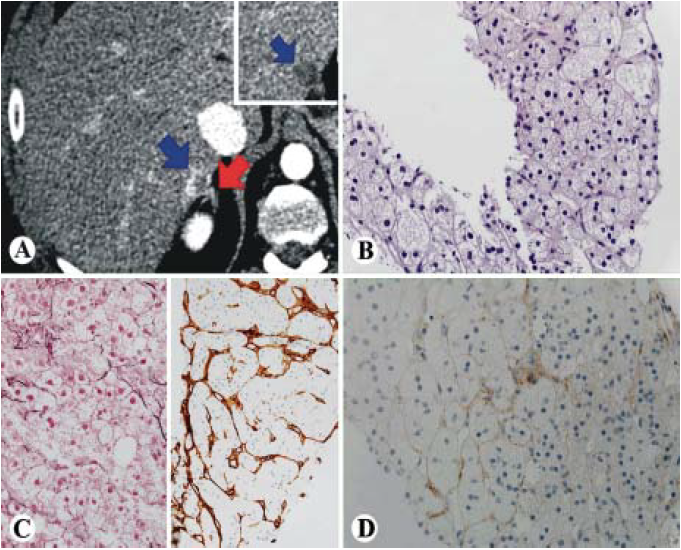
Figure 1 Foamy histiocyte-like HCC (case 1). A. CT scan showing a 1.3 cm mass that enhances during the arterial phase (blue arrow) with partial washout on the delayed images (inset). The adrenal gland is in close proximity to the lesion (red arrow). B-D. Histologically, the tumor is well differentiated HCC with foamy cytoplasm and low N/C ratio (B, HE, 400x), loss of reticulin framework (C, left, 400x), positive diffuse sinusoidal staining of CD34 (C, right, 200x) and negative for glypican 3 (D, 400x).
A partial resection was attempted, but was aborted due to the presence of micronodularity, severe steatosis and subsequent concern for decompensation. The patient is alive 6 months after diagnosis and is under consideration for transplantation after successfully undergoing two thermal ablation treatments.
Case 2
A 77-year old male was referred to our institution to follow up on elevated liver enzymes and a suspicious mass on ultrasound at an outside institution. He had a 40-pack year smoking history but quit in 2000, drank alcohol occasionally, and used no recreational drugs. He had no known family history of liver disease. His labs demonstrated the following: white blood cell count was 5,900/μL, hemoglobin was 13.8 g/dL, Platelets count was 19.3 x 104/μL, total.bilirubin was 0.7 mg/dL, albumin was 3.7 g/dL, aspartate aminotransferase was 86 IU/L, alanine aminotransferase was 112 IU/L, alkaline phosphatase was 115 IU/L, alpha-fetoprotein was 4.3 ng/mL, Carcinoembryonic antigen was (CEA) 1.4 ng/mL, CA 19-9 was 46 IU/mL, prothrombin time was 14.8 seconds, and INR was 1.2. Hepatitis C antibody was reactive, and Hepatitis C RNA by quantitative PCR of 6.1 log IU. Hepatitis B surface antibody was reactive, Hepatitis B surface antigen non-reactive, and Hepatitis B core antibody IgM non-reactive. On initial visit at our institution he was asymptomatic and physical exam was unremarkable. A CT in October 2014 showed a large heterogeneously enhancing liver mass during the arterial phase with partial washout and a pseudocapsule in the venous phase. A tumor thrombus is readily visible in the inferior vena cava with venous washout (Figure 2A). There was also some background liver disease noted. HCC was the initial concern, but colon cancer metastasis or cholangiocarcinoma were also on the differential. Also a CT chest revealed centrilobular emphysema with a 7 mm pulmonary nodule. A bone scan was negative for metastases. A core needle biopsy of the liver demonstrated a tumor composed of irregular arrangement of hepatocytes with increased hepatic plate thickness. The neoplastic cells had a low nuclear to cytoplasmic ratio and abundant foamy pale cytoplasm (Figure 2B). There was a loss of reticulin framework by reticulin stain (Figure 2C left) and diffuse pattern of sinusoidal CD34 immunostaining (Figure 2C right). Tumor cells were positive for glypican 3 (Figure 2D). PASD was negative for alpha-1- antitrypsin globules. The patient was not a surgical candidate due to large tumor size, cirrhosis, portal hypertension and poor general condition. He was started on Sorafenib as a chemotherapeutic agent. The patient's cancer became metastatic, with extension into the renal veins, inferior vena cava and right atrium. He was referred for hospice care one month later.
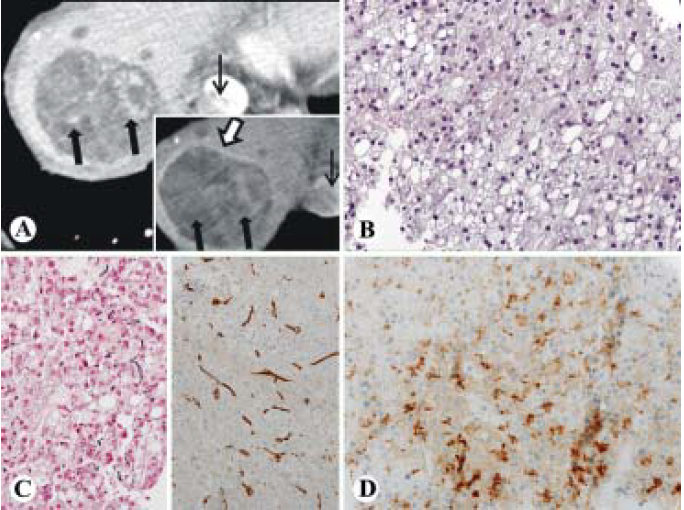
Figure 2 Foamy histiocyte-like HCC (case 2). A. CT images demonstrating a large heterogeneously enhancing liver mass during the arterial phase (thick arrows) with partial washout (inset, thick arrows) and a pseudocapsule (inset, open arrow) in the venous phase. A tumor thrombus is readily visible in the inferior vena cava (thin arrow) with venous washout (inset, thin arrow). B-D. Histologically, the tumor is well differentiated HCC with foamy cytoplasm and low N/C ratio (B, HE, 400x), loss of reticulin framework (C, left, 400x) positive diffuse sinusoidal staining of CD34 (C, right, 200x) and positive for glypican 3 (D, 400x).
DISCUSSION
Among men, hepatocellular carcinoma is the second leading cause of cancer death worldwide, and sixth in the U.S.1 In the U.S. it is most often associated with cirrhosis due to hepatitis C, hepatitis B or alcohol. Diagnosis usually comes from screening individuals with these risk factors and is often made via imaging techniques. For those diagnosed, prognosis is poor with mortality occurring in about 6 to 20 months.2 Carcinomas with foamy histiocytelike appearance have also been reported in breast and pancreas tumors, and their outcomes are not well known.3 - 5 HCC with foamy histiocyte-like appearance may be under recognized.6 The outcome of this entity is unknown. We therefore report two cases and discuss the diagnostic challenges.
Foamy histiocyte-like type hepatocellular carcinoma is a rarely reported subtype of HCC that, to the best of our knowledge, has only been reported once in the English literature.6 Here, we present two cases, one in a 49 year old male with hepatitis C, and the other in a 77 year old male also with hepatitis C. One reason for the rarity of this entity is the similar histological appearance to clear cell HCC. In the clear cell variant of HCC, cells retain glycogen or fat, causing the clear cell appearance on routine sections. These contents dissolve during processing, leaving a clear space behind. Foamy histiocyte-like HCC has foamy cytoplasm and may also be differentiated from clear cell HCC by glycogen or mucin staining. Absence of staining for CD 68 can be used to determine that the cells are not histiocytes. Additionally, Hep-Par-1 or arginase-1 can be used to distinguish whether or not the cells are of hepatic origin.6 Studies in foamy gland pattern pancreatic ductal carcinoma suggest that the foamy appearance in that tumor is due to accumulation of vacuolated, potentially altered mucigen granules.4
This subtype of HCC presents a diagnostic challenge for radiologists and pathologists. Radiologically, the majority of conventional HCCs can be diagnostic by imaging findings with a washout appearance as in Milan criteria. However, these two new cases and the previously reported case presented in an atypical fashion on dynamic 3 phase CT with partial (heterogeneous) arterial phase enhancement and partial venous phase “washout”, compared to the typical presentation of HCC with homogenous enhancement and washout respectively. In pathology, the histological characteristics of conventional well-differentiated HCCs are increased cell density, increased nuclear to cytoplasm ratio, an irregular trabecular pattern, increased eosinophilic or basophilic staining intensity, increased acinar or pseudoglandular pattern, and fatty change or clear change of the cancer cells.7 However, these tumors appeared well-differentiated, with no increased nuclear to cytoplasm ratios and no significant nuclear atypia, but were positive for diffuse sinusoidal staining pattern of CD34 and focally positive for glypican 3, as well as showing loss of reticulin, giving them a diagnosis of well-differentiated HCC despite their benign foamy cytologic appearance.
The outcome of hepatocellular carcinoma with foamy histiocyte-like features is unknown based on the paucity of available data. Though rare, there are other examples of this type of lesion. One is a histological variant of pancreatic ductal adenocarcinoma known as foamy gland pattern type. These show microvesicular change in the cytoplasm similar to the lesions reported here, but differ in that they have nuclear atypia, hyperchromatic nuclei, and the microvesicular pattern is basally located rather than diffuse. The median survival rate for these patients was not significantly different from those with ordinary ductal adenocarcinoma.4 Foamy cells are also found in prostatic carcinoma as a known variant. In these cases, abundant foamy cytoplasm is found amidst crowded glands, pink acellular secretions, and without enlarged nuclei or prominent nucleoli.8 This subtype also exists as a rare subtype of breast carcinoma featuring large, clear to foamy cytoplasm with cellular pleomorphism and nuclear atypia. Due to the rarity of these lesions, the aggressiveness has not been well studied.5 The outcome in our cases is varied. The patient in case 1 is now nearly 24 months post diagnosis and is being considered for liver transplant after two microwave ablations treatments of his lesion. As of February 2016 follow up, there was no evidence of residual tumor, though it is not considered cured. The patient in our case 2 presented with a more advanced tumor in October 2014. He was not a surgical candidate, and after a one month course of Sorafenib, was recommended to hospice care owing to progression to stage IV disease and he died a few weeks later. In the only other known documented case of foamy histiocyte-like HCC, the patient presented with a 45 mm mass which was successfully resected. As of the time of publication, the patient had survived 2.5 years without recurrence. Given these scenarios, wherein two of the three patients' survival was longer than 20 months from discovery, it is possible this cancer subtype has better outcomes than average prognosis for conventional hepatocelluar carcinoma, however, more cases of such subtype HCC are needed.
In summary, foamy histiocyte-like variant of hepatocellular carcinoma is extremely rare and challenging to diagnose both radiologically and pathologically. In these three known cases, it has presented as a well-differentiated, low-grade tumor that may have relatively positive outcomes for the afflicted patients, but further cataloguing is needed for reliable data.











 text new page (beta)
text new page (beta)


