INTRODUCTION
Intrahepatic cholestasis of pregnancy (ICP) is a severe liver disease that uniquely occurs during pregnancy, and it is often referred to as jaundice in pregnancy, obstetric cholestasis, hepatosis gestationalis, or obstetric hepatosis.1 ICP is commonly characterized by pruritus, which starts in the second or third trimester during pregnancy and withdraws quickly after delivery.2 In approximately one out of 10 cases, mild jaundice usually occurs within one month after the start of itching.3 , 4 Other symptoms, though not typical, includes subclinical steatorrhea which may cause deficiency in vitamin K,5 , 6 abdominal pain and encephalopathy.7 Ever since its first description in 1883, ICP is not commonly treated as a serious clinical problem, even though there is increased risk of sudden intrauterine fetal death and preterm delivery.8
Prevalence of ICP varies with both ethnic origin and geographic location.1 , 8 , 9 The highest reported incidence to date was in Chile, Bolivia and Scandinavia, which was as high as 15%.2 , 10 The high incidence rate of ICP in the above countries was thought to be caused by both dietary and environmental factors,2 however the prevalence was found to be greatly reduced in later studies.10 , 11 On the other hand, ICP incidence in Asia has increased.12 , 13 ICP has also gained growing clinical attention in China, due to its high prevalence in Chinese population recently.14 , 15
To date the exact cause of ICP still remains largely unknown, except that the pathogenesis of ICP is believed to be multifactorial, resulting from environmental influences, nutritional deficiencies, genetic predisposition and variations as well as changes in hormone levels.16 Such a multifactorial nature makes the diagnosis and treatment of ICP difficult and problematic. Early studies have found that during ICP the flux of total bile acids (TBA) was increased from the mother to the fetus,17 - 19 and high maternal TBA level could disturb hormone production and transport in the placenta, as well as chorionic vessel constriction.20 Therefore elevated serum TBA level was considered as the most sensitive laboratory sign of abnormality in ICP. Serum level of TBA above 11.0 μM was also considered to be the most accurate early diagnostic marker of ICP,21 while the most widely accepted diagnostic end point for ICP is from 10 to 14 μM. Based on TBA levels, ICP can be categorized into mild ICP with TBA from 10 to 40 μM, and severe ICP with TBA above 40 μM.22 However, serum TBA level also varies depending on the study population,16,22 making the clinical use of TBA insufficient for biochemical diagnosis.23
In this study, we aimed to analyze the characteristics of ICP in Chinese patients.
MATERIALS AND METHODS
Patients
From 2010 to 2014 in Wuxi Maternity and Child Health Hospital Affiliated to Nanjing Medical University, 98 women with ICP and 50 healthy pregnant women, all in their last trimester of pregnancy, participated in the study. 30 healthy non-pregnant women were also recruited in the study to better define diagnostic criteria. Informed and written consent was acquired from all participants and this current study followed the ethical guidelines of the Declaration of Helsinki of 1975. This study was approved by the ethics committee of Wuxi Maternity and Child Health Hospital Affiliated to Nanjing Medical University.
The inclusion criteria were:
Pruritus and/or jaundice.
Absence of dermatological disease except lesions due to excessive scratching.
Serum TBA concentration higher than 15 μM.
Absence of current viral hepatitis.
Absence of chronic liver disorder or symptomatic cholelithiasis.
Absence of biliary tract dilatation on ultrasound examination.
Normalization of routine liver function tests following delivery.
No signs of fever, pre-eclampsia, endocervical or urinary infection.
Among the 98 ICP patients, 50 were further categorized as mild ICP using previously reported grading criteria:24
Total bilirubin (TBIL) < 21 μM, direct bilirubin (DBIL) < 6 μM.
Alanine transaminase (ALT) < 250 U/L, aspartate aminotransferase (AST) < 150 U/L.
Cholyglycine (CG) < 30 mg/L.
The rest 48 patients who have met any of the following criteria were categorized as severe ICP:
TBA concentration was not used as grading criteria. All the following analyses were conducted by an investigator blind to the ICP patient category.
Liver function tests
Commercially available enzyme-linked immunosorb ent assay (ELISA) kits (Sigma-Aldrich) were used to perform liver function tests following manufacturers’ protocols, including serum TBIL, DBIL AST, ALT and CG, as previously described.21 Upper limits of normal values in our study for non-pregnant women were 15 μM for TBIL, 5 μM for DBIL, 40 U/L for ALT, 40 U/L for AST, 3 mg/L for CG and 6 μM for TBA, respectively.
Blood sample analysis
Venous blood sample of patients were collected following an overnight fast. The serum was separated by centrifugation immediately and stored at -80 °C until blood biochemical parameter analyses were performed. Serum TBA level was measured using an established enzymaticfluorimetric assay25 ) after solid-phase extraction with SepPak C18 cartridges.26 ) Serum levels of matrix metalloproteinase (MMP)-2 and MMP-9 were determined with the Human MMP-2 and MMP-9 ELISA kits (Sigma-Aldrich) following manufacturers’ protocols.
Statistical analysis
Data were expressed as mean ± SEM. Comparison of results were performed using unpaired and two-tailed Student’s t-test, or ANOVA analysis followed by Tukey’s post hoc test, on the basis of equal or unequal variance as appropriate. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software (SPSS Inc., USA).
RESULTS
Clinical characteristics
From 2009 to 2014, among a total of 4,137 pregnancy cases admitted in our hospital, 172 were diagnosed with ICP, making the ICP incidence of 4.16%. Among the 172 ICP patients, 98 participated in this study based on the inclusion criteria. Using previously reported grading criteria,24 ) 50 of the participants were catergorized as mild ICP and the rest 48 were catergorized as severe ICP. Clinical characteristics in healthy pregnant women, mild and severe ICP women were summarized in Table 1. Statistical differences were observed with respect to gestational age at full term and newborn birth weight (P < 0.05), not only between healthy pregnant women and mild ICP women, but also between mild and severe ICP women. Labor was induced in 58% of both mild and severe ICP groups, which was markedly higher than healthy pregnant women with only one case of induced labor. Taken together, the clinical characteristics among the three groups of participants provided reliable grading to distinguish healthy, mild ICP and severe ICP pregnant women.
Table 1 Clinical characteristics in healthy pregnant women and patients with mild and severe ICP.
Results were compared using unpaired two-tailed Student’s t-test, performed on the basis of equal or unequal variance as appropriate. Values are mean ± SEM. N.A. not applicable. * P < 0.05 compared to healthy pregnant women; † P < 0.05 compared to mild ICP women.
Liver function tests
We next performed liver function tests during the third trimester, including TBIL, DBIL, ALT, AST and CG, in healthy pregnant women, mild and severe ICP women.
The results were summarized in Table 2. For healthy pregnant women, all test results were within normal range of the third trimester during normal pregnancy. Both mild and severe ICP women exhibited significant elevations (P < 0.05) for all tests comparing to healthy pregnant women. Moreover, all test results were also found to be significantly elevated (P < 0.05) in severe ICP women compared to mild ICP women.
Table 2 Liver function tests during the third trimester of healthy pregnant women and patients with mild and severe ICP
TBIL: total bilirubin. DBIL: direct bilirubin. ALT: alanine transaminase. AST: aspartate aminotransferase. CG: cholyglycine. Results were compared using unpaired two-tailed Student’s t-test, performed on the basis of equal or unequal variance as appropriate. Values are mean ± SEM. * P < 0.05 compared to healthy pregnant women; † P < 0.05 compared to mild ICP women.
Serum TBA levels
Several reports performed in other ethnic groups suggested that an elevation in serum TBA levels could be used as the most sensitive laboratory sign of abnormality in ICP.17 - 19 ) We therefore investigated whether this criterion could be applied to Chinese ICP patients. Venous blood samples were collected from all participants following an overnight fast, and serum TBA concentrations were measured. The results were summarized in figure 1. In general, TBA concentrations in healthy pregnant women were progressively lower than those of both mild and severe ICP women as well. However, to our surprise, the differences were not statistically significant, which suggested serum TBA might not serve as a laboratory abnormality sensitive enough for the diagnosis of ICP in Chinese population.

Figure 1 Serum total bile acids (TBA) levels in healthy pregnant women and patients with mild and severe ICP. Results were compared using unpaired two-tailed Student’s t-test, performed on the basis of equal or unequal variance as appropriate. Values are mean ± SEM. ns1, not statistically significant compared to healthy pregnant women; ns2, not statistically significant compared to mild ICP women.
Serum matrix metalloproteinase (MMP)-2 and 9 levels
Since a more sensitive laboratory test is needed for accurate diagnosis and grading of ICP in Chinese population, we went on to test several other candidate factors that could be easily and accurately measured in blood samples, including MMP-2 and MMP-9. As shown in Figure 2A, serum levels of MMP-2 in mild ICP patients were significantly higher (P < 0.05) than that of healthy pregnant women, while MMP-2 serum levels of severe ICP patients were also significantly elevated (P < 0.05) than that of mild ICP patients. Nevertheless, serum levels of MMP-9 also exhibited the same progressive trend of elevation as MMP-2 in all three groups of participants (Figure 2B). Our data hereby strongly indicated that, in Chinese population, serum levels of MMP-2 and MMP-9 could serve as a more sensitive diagnostic factor for ICP.
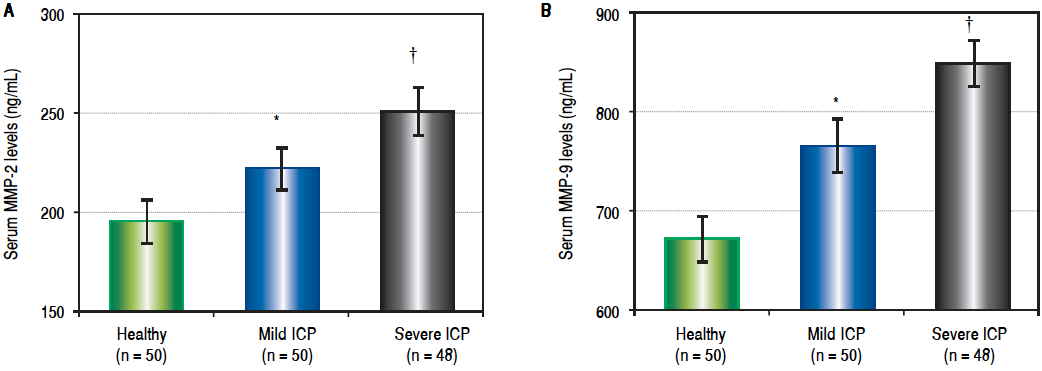
Figure 2 Serum MMP-2 (A) and MMP-9 (B) levels in healthy pregnant women and patients with mild and severe ICP. Results were compared using unpaired two-tailed Student’s t-test, performed on the basis of equal or unequal variance as appropriate. Values are mean ± SEM. * P < 0.05 compared to healthy pregnant women; † P < 0.05 compared to mild ICP women.
Positive correlation between serum levels of MMPs and liver function tests
Furthermore, we tested whether the increase in serum levels of MMP-2 and MMP-9 was correlated to liver function tests, which were used as grading factors of ICP patients. As summarized in table 3, we observed consistent and significant positive correlations between increases in MMP-2 and MMP-9 levels with all liver function test parameters (γ > 0.5), with the exception of DBIL (γ <0.4). In contrast, we didn’t observe any strong linear correlations between TBA and liver function tests (data not shown).These results have clearly suggested that serum levels of both MMPs could be reliably used as laboratory abnormalities for accurate diagnosis of ICP.
DISCUSSION
In our current study, we have examined a widely used known diagnostic marker, serum TBA concentration, in both mild and severe ICP patients, compared with healthy pregnant women. Although several previous reports conducted on ICP women from other ethnic groups suggest ed that elevated serum TBA levels could serve as an laboratory sign of abnormality in ICP, ( 17 - 19 this isn’t the case in our study, which focused entirely on Chinese patients. Our results indicated a progressive increase in serum TBA concentrations, by comparing serum TBA in healthy pregnant women with that of mild ICP patients, as well as severe ICP patients. However, this trend of increase in serum TBA was not statistically significant, therefore making its use as a laboratory abnormality to diagnose and grade the severity of ICP questionable among Chinese patients. For years our hospital and others have been relying on liver function tests, such as TBIL, DBIL, ALT, AST and CG, combined with the typical symptoms of pruritus and/or jaundice, for the diagnosis and grading of ICP patients.24 Although compared with TBA, liver function tests were more reliable to diagnose Chinese ICP patients, the procedures are lengthy and expensive. Other serum diagnostic markers that are sensitive, accurate and easily measurable are desperately needed.
MMPs are extracellular matrix-degrading enzymes secreted by the placenta during tissue remodeling, among which both MMP-2 and MMP-9 were found to be implicated in various diseases during pregnancy. For instance, low maternal levels of serum MMP-2 was reported to be associated with fetal inflammatory response and preterm labor,27 whereas high maternal levels of serum MMP-2 was found in patients of peripartum cardiomyopathy.28 In pregnant women with gestational diabetes mellitus (GDM), the level of TIMP-2, a tissue inhibitor of MMP 2, was found to be significantly higher, whereas ratio of MMP-2/TIMP-2 was significantly lower in GDM patients.29 Synthesis of MMP-2 and MMP-9 was changed in pre-eclampsia.30 , 31 Moreover during impaired placental development, secretion of both MMP-2 and 9 was reduced, leading to intrauterine growth restriction.32 Aberrant increase in MMP-2 and 9 activities in the amnion of fetal membranes was also found to be correlated to membrane premature ruptures.33 The above studies collectively suggested that levels and activities of both MMP-2 and 9 are tightly regulated during pregnancy, and their disrup tions usually result in a wide spectrum of maternal and fetal symptoms. In this context, we have examined serum levels of MMP-2 and MMP-9 in ICP patients. Our results indicated that serum levels of both MMPs are consistently upregulated in ICP patients, compared with healthy pregnant women. Moreover, even among ICP patients, their serum levels of both MMPs were found to be sensitive enough to reflect the severity of their disease, as evident by strong and positive correlations of both MMP levels with results of liver function tests. Nevertheless, as the sample population of our current study was focused entirely on Chinese population, further studies are needed to validate whether MMP-2 and MMP-9 could also serve as markers for clinical diagnosis of ICP.
CONCLUSION
Results from our current study have advised against the use of serum TBA level in Chinese population to diagnose ICP. More importantly, we have also shown for the first time that in Chinese population, serum levels of both MMP- 2 and MMP-9 could be reliably used as laboratory abnormalities for accurate diagnosis and sensitive grading of ICP.











 nueva página del texto (beta)
nueva página del texto (beta)


