INTRODUCTION
Portal vein thrombosis (PVT) is not uncommon in candidates undergoing liver transplantation (LT) presenting a unique set of challenges. The prevalence of PVT in cirrhotic patients at evaluation or at the time of transplantation varies from 5% to 26%.1 - 3 The majority of patients have partial thrombosis. In cirrhotic patients, the increase in intrahepatic vascular resistance and decreased portal blood flow are likely predisposing factors for PVT. The prevalence of PVT in candidates for LT seems to be similar to that found in cirrhotic patients who were not necessarily evaluated for transplantation.4 , 5 A large proportion of patients with PVT at the time of surgery are previously unrecognized (up to 50%).6
While there have been significant improvements in surgical techniques, reports are conflicting on whether post-LT mortality in PVT patients is equivalent to nonPVT patients.7 - 9 Medical (anticoagulation/TIPS) and surgical measures aim to preserve/restore portal flow in the pre/perioperative period so that anatomical end-to-end portal anastomosis can be performed. More recent literature suggests that subgroups of PVT patients that may have worse outcomes post-LT or have higher rates of posttransplant complications include patients with pre-operative complete/extensive thrombosis and those left with non-physiologic portal flow post LT.10
Anticoagulation in PVT has been examined in a few small studies (19-26 patients). The vast majority of patients included in these analyses had partial/incomplete thrombosis with complete recanalization rates varying between 40-75%.6 , 11 , 12 Currently there is no consensus regarding which agent is best. However vitamin K antagonists (warfarin) impact INR in calculations of MELD whereas low molecular weight heparin (LMWH) does not. While there are no current accepted hematological parameters (e.g. INR, thromboelastography) relating to safety of initiating anticoagulation, reported variceal bleeding and intraoperative bleeding rates are similar to non-anticoagulated cirrhotic patients.6
While most studies have focused on surgical issues (e.g. thrombosis in living donor) and anticoagulation there are potentially other factors that may impact outcome post-LT in PVT patients.
In this multicenter Canadian retrospective cohort study, our aims were as follows:
Explore the impact of PVT along with other factors which impact post-LT outcomes.
Determine what factors are associated with worse post-LT outcomes amongst PVT patients.
Determine the prevalence and impact of pre/post-LT use of antiplatelet agents and anticoagulation on postLT outcomes for patients with PVT.
Using multivariable analysis, determine what patient and transplant factors are associated with worse postLT outcomes in PVT patients.
MATERIAL AND METHODS
The reporting of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.13 The local health research ethics board approved this study and the requirement for individual informed consent was waived. The ethical principles of the Declaration of Helsinki for medical research involving human subjects were fulfilled.14
Design, setting, participants, and data collection
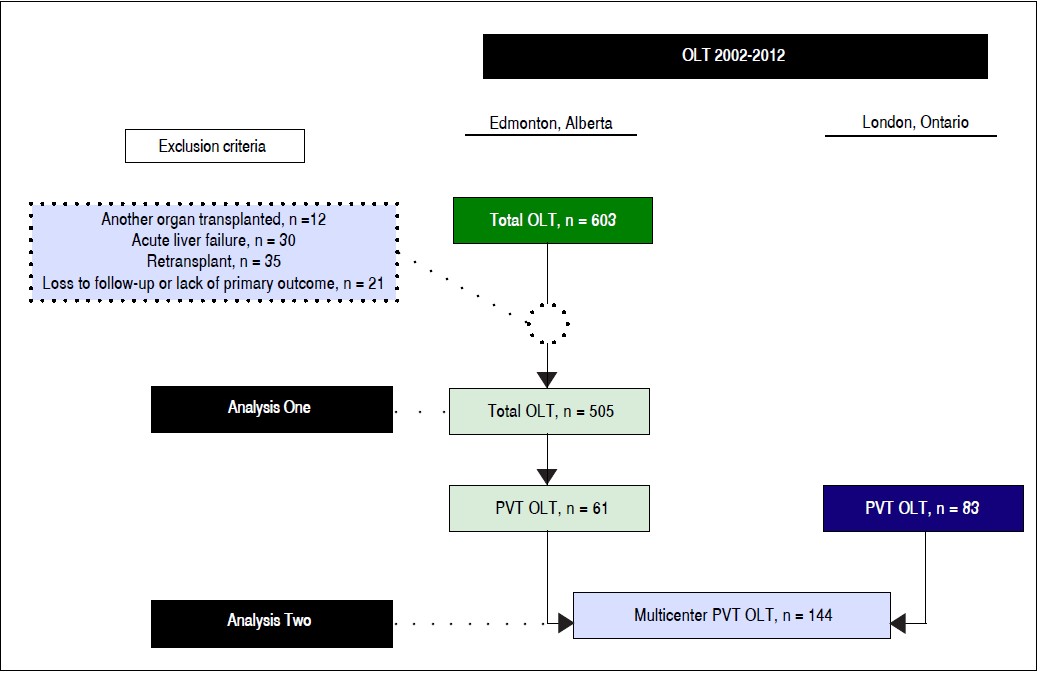
Analysis One (single center) was a retrospective cohort study of adult (age ( 18 years) patients with end-stage liver disease who underwent primary LT at the University of Alberta Hospital (Edmonton, Alberta, Canada) between January 1, 2002 and June 30, 2012 (Figure 1). Patients were excluded if:
They were concomitantly transplanted with another organ.
Their primary diagnosis was acute liver failure.
They were receiving a re-transplant.
They were lost to follow-up, or
There was missing data on the primary outcome.
Included in this dataset were the cohort of patients with PVT diagnosed prior to or at LT. Data on patients’ baseline characteristics were retrieved from a dedicated computerized database and individual medical records. The data included: age, sex, body mass index, indication for LT, complications of cirrhosis, comorbidities, Intensive Care Unit (ICU) admission immediately prior to LT, severity of liver disease indexes (Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease (MELD)) at LT, laboratory tests at LT, live donor transplant, donor risk index,15 and portal vein status at LT. Given the results found in Analysis One (PVT in 14% of patients), we decided to add data from another LT center to enhance statistical validity of PVT patients specific analysis, but as this was incomplete for the purpose of Analysis One, we obtained such additional data only for Analysis Two, thus minimizing selection bias. Therefore, Analysis Two consisted of a multicenter cohort study of cirrhotic patients with PVT diagnosed previously to or at LT from the University of Alberta Hospital and London Health Sciences Center (London, Ontario, Canada) during the same time period (January 1, 2002 and June 30, 2012) (Figure 1). For PVT patients, the following data was additionally collected: PVT characteristics, any other hypercoaguable state (e.g. myeloproliferative disease, factor V Leiden deficiency, S or C protein deficiency, antithrombin deficiency), preand post-LT antithrombotic therapy, portal vein surgical approach, red-blood cells transfusions during transplant, and portal vein reocclusion after LT.
Operational definitions
Cirrhosis was defined as bridging fibrosis on previous liver biopsy or a composite of clinical signs and findings provided by laboratory tests, endoscopy, and radiologic imaging.16 Pretransplant complications of cirrhosis considered were infection, variceal bleeding, hepatic encephalopathy, hepatorenal syndrome, and hepatopulmonary syndrome. Definitions for these complications are provided in Supplementary Materials and Methods. Pretransplant comorbidities considered were coronary artery disease, chronic pulmonary obstruction, diabetes mellitus, and chronic kidney disease. Definitions for these comorbidities are also provided in Supplementary Materials and Methods. MELD was calculated according to the United Network for Organ Sharing (UNOS) recommendations,17 without adjusting for serum sodium or standardized exception points.18 PVT was diagnosed by standard imaging techniques, generally abdominal ultrasound Doppler, abdominal contrast-enhanced computerized tomography, and/or abdominal contrast-enhanced magnetic resonance.19 The surgical approach to PVT during LT included:
Eversion thrombectomy and end-to-end anastomoses.
Interpositional vein grafting, if the anastomosis between the recipient splenomesenteric confluence and the donor portal vein is possible.
Mesoportal jump grafting, if the thrombus extends below the splenomesenteric confluence but the proximal superior mesenteric vein is patent, or
Venous reconstruction, if none of the previous techniques is feasible.10
Primary exposure and outcome
For Analysis One (single center), the primary exposure was PVT and primary outcome was post-LT overall mortality. For Analysis Two, amongst PVT patients from two sites, stratification was made between survivors and nonsurvivors to determine which covariates were associated with post-LT overall mortality.
Statistical analysis
Categorical variables (Analysis One and Two) were presented as proportions and continuous variables as mean and standard deviation (SD), if normally distributed, or median and inter-quartile range (IQR), if non-normally distributed. Univariate comparisons of patients’ baseline characteristics were performed with χ2, Student t, or Mann-Whitney tests where appropriate.
In Analysis One (Edmonton cohort), cumulative survival was studied using Kaplan-Meier curve and Log-rank test and adjusted survival was modelled using Cox proportional hazard regression analysis. In Analysis Two (multicenter cohort), survival was then modeled with Cox proportional hazards regression. As the rate of missing values for this cohort was greater than 5%, multiple imputation (5 iterations) was performed in order to use the complete set of patients. Variables with a P < 0.05 in the univariate analysis were initially included in the model. No interactions were observed. A backward stepwise automatic process (Wald statistic) was then used to derive the best final model, which was subsequently internally validated with bootstrapping (1,000 replications). The final model’s performance was evaluated by χ2. Statistical analysis used IBM SPSS Statistics, version 20 (IBM Corp, North Castle, NY).
RESULTS
Analysis One: Descriptive characteristics of 505 consecutive LT patients from Edmonton
A total of 505 patients met the eligibility criteria. Median age was 54 (IQR, 49-59) years and 68% (344/505) were men. Hepatitis C and hepatocellular carcinoma were responsible for 25% (124/505) and 22% (109/505), respectively, of all indications for LT. The most common complications of cirrhosis prior to LT were variceal bleeding [63% (229/366)], hepatic encephalopathy [61% (308/505)], and infection [41% (184/452)]. Median CTP and MELD scores at LT were 10 (IQR, 8-12) and 15 (IQR, 11-23), respectively. ICU stay immediately prior to LT was necessary for 13% (64/505) of patients. Living donors were used in 13% (64/505) of patients. Median overall follow-up time was 1302 (IQR, 544-2,340) days. All baseline characteristics for this cohort are shown in table 1.
Table 1 Analysis One: Baseline characteristics of 505 liver transplant recipients from Edmonton (01/2002-06/2012) stratified by portal vein thrombosis status.
n: frequency. IQR: interquartile range. SD: standard deviation. PVT: portal vein thrombosis. LT: liver transplant. COPD: chronic obstructive pulmonary disease. CKD: chronic kidney disease. GFR: glomerular filtration rate. INR: international normalized ratio. CTP: Child-Turcotte-Pugh. MELD: Model for End-Stage Liver Disease. ICU: Intensive Care Unit. *Total number of patients with known PVT status was 447 out of 505 (89%).
Analysis One: Univariate comparisons between PVT and non-PVT patient in the Edmonton cohort
Baseline characteristics of PVT and non-PVT patients are presented in table 1. PVT was present in 14% (61/447) of patients where PVT status was known (PVT status could not be determined in 58 (11%) patients who were excluded from univariate analysis). When considering two different time periods (2002-2006 vs. 2007-2012), PVT was diagnosed in 12% (21/182) and 15% (40/265) of patients, respectively. In comparing PVT patients (n = 61) and patients without known PVT (n = 386), there were no significant differences in rates of infection, variceal bleeding, hepatic encephalopathy, or hepatorenal syndrome prior to LT (p > 0.20 for all comparisons). Laboratory parameters were similar on the date of LT (p > 0.05 for all comparisons). PVT and non-PVT patients had also similar CTP (10 vs. 10) and MELD scores (15 vs. 16) on the day of LT (p > 0.20 for both comparisons).
Analysis One: Outcomes amongst PVT and non-PVT patients in the Edmonton cohort
Within 505 patients in the single center cohort, overall post-transplant mortality during follow-up time was 27% (134/505). Causes of death were recurrent underlying liver disease (33%; n = 44), sepsis (24%; n = 32), cardiovascular events (18%; n = 24), de novo malignancy (11%; n = 15), chronic rejection (3%; n = 4), and others (11%; n = 15). There were a total of 18 retransplants (Table 1: 2 PVT, 13 non-PVT, and 3 unknown PVT status) within the follow-up period. Causes of these graft failures were hepatic artery thrombosis (10, including 3 with concomitant PVT), primary nonfunction (3), biliary duct dysfunction (1), acute rejection (1), chronic rejection (1), recurrent underlying liver disease (1), and unspecified organ ischemia (1). Figure 2 compares unadjusted overall survival stratified for PVT and non-PVT patients. PVT status was not associated with a significant survival difference between groups (Log-rank test, p = 0.99). When considering two different time periods (2002-2006 vs. 2007-2012), overall mortality amongst PVT patients decreased (15 vs. 8%, respectively), but in both time periods PVT was not associated with overall mortality (p > 0.20 for both comparisons). Re-transplant rate was similar between PVT and non-PVT patients (3 vs. 3%, p = 0.98). Median length of LT hospital stay was also similar between PVT and non-PVT patients (Table 1: 22 vs. 24, p = 0.68).
Analysis One: Multivariable analysis for overall mortality (Edmonton)
Results of Cox proportional hazards modelling for the single center cohort are shown in table 2. Two separate models were derived; one including PVT and one with complete/occlusive PVT. In model one, PVT was not associated with overall mortality on unadjusted [Hazard Ratio (HR) 1.00 (0.57-1.75); p = 0.99] or adjusted analysis [HR 0.39 (0.05-3.07); p = 0.19] after including other covariates. However, in Model 2, the presence of complete/ occlusive PVT was significantly associated with increased overall mortality [HR 8.42 (2.10-33.7); p = 0.001] after adjusting for other covariates.
Table 2 Analysis One: Cox proportional hazards regression study of the association of portal vein thrombosis and covariates with overall mortality (Edmonton cohort).
HR: hazard ratio. CI: confidence interval. PVT: portal vein thrombosis. MELD: Modified End-stage Liver Disease. DRI: donor risk index. * Model 1: n = 151; χ 2 8. ** Model 2: n = 56; χ 2 13. *** P after bootstrapping (1,000 samples).
Analysis Two: Multicenter analysis of 144 consecutive PVT patients who underwent LT (Edmonton and London)
A total of 144 patients with PVT were included, 61 from the University of Alberta Hospital and 83 from the London Health Sciences Center. Median age was 55 (IQR, 50-61) years and 65% (94/144) were men. Hepatitis C and hepatocellular carcinoma were responsible for 26% (37/144) and 8% (11/144), respectively, of all indications for LT. The most common complications of cirrhosis previously to transplant were hepatic encephalopathy [58% (84/144)], variceal bleeding [49% (68/138)], and infection [30% (42/141)]. Median CTP and MELD scores at LT were 10 (IQR, 8-11) and 16 (IQR, 12-25), respectively. ICU stay immediately prior to LT was necessary for 15% (21/144) of patients. A liver from a live donor was used in 8% (12/144) of patients.
Complete/occlusive PVT was present in 20% (12/61) of patients where data were available. Anticoagulants were used by 7% (4/144) of patients before LT. Portal vein recanalization occurred in 30% (18/61) of patients. Surgical thrombectomy was performed in 39% (24/61) of patients. Anticoagulants were used by 62% (89/144) of patients following LT. Post-transplant portal vein reocclusion occurred in 6% (9/144) of patients. From the 9 patients with post-LT portal vein re-occlusion, 7 were on anticoagulation and 2 were not (8 vs. 4%, p = 0.48). All baseline characteristics for this cohort are shown in table 3.
Table 3 Analysis Two: Baseline characteristics of the multicenter cohort (Edmonton and London, 01/2002-06/2012) stratified by overall survival status.
n: frequency. IQR: interquartile range. SD: standard deviation. LT: liver transplant. CKD: chronic kidney disease. GFR: glomerular filtration rate. INR: international normalized ratio. CTP: Child-Turcotte-Pugh. MELD: Model for End-Stage Liver Disease. ICU: Intensive Care Unit. PVT: portal vein thrombosis. OR: operating room. PVT: portal vein thrombosis. ASA: acetylsalicylic acid.
Analysis Two: Characteristics associated with overall mortality amongst PVT patients (Edmonton and London)
Demographic, biochemical and descriptive information about PVT regarding the combined cohort of 144 consecutive PVT patients (Alberta and London) are shown in table 3. Median overall follow-up time was 1,050 days (IQR, 367-1840). Overall post-transplant mortality during follow-up time was 21% (30/144). On univariate comparisons (Table 3), non-survivors were more likely to have hepatitis C (40 vs. 22%, p = 0.04). Pre-LT PVT characteristics (n = 61 Alberta only) were similar between nonsurvivors and survivors with the exception of complete thrombosis (43 vs. 13%, p = 0.01). Perioperative characteristics were similar except that surgical thrombectomy was required more frequently in non-survivors (64 vs. 32%, p = 0.03). Post-LT portal vein re-occlusion occurred more frequently in non-survivors than survivors (17 vs. 4%, p = 0.02). The presence of a hypercoaguable state and anticoagulation use before or following LT were not associated with survival status (p > 0.20 for both comparisons).
Analysis Two: Multivariable analysis for overall mortality (Edmonton and London)
Cox proportional hazards regression was used to determine independent characteristics associated with overall mortality amongst PVT patients. On unadjusted analysis, while hepatitis C [HR 2.2 (1.0-4.5); p = 0.04], post LT portal vein re-occlusion [HR 3.2 (1.2-8.4); p = 0.02], and complete/occlusive PVT [HR 3.7 (1.3-10.7); p = 0.02] were associated with increased post-transplant overall mortality, requirement for surgical thrombectomy [HR 2.6 (0.9-7.8); p = 0.09] was not. On adjusted analysis, only hepatitis C [HR 2.1 (1.0-4.5); p = 0.03] and post-transplant portal vein re-occlusion [HR 3.2 (1.2-8.3); p = 0.01] remained significantly associated with post-transplant overall mortality (Table 4).
Table 4 Analysis Two: Cox proportional hazards regression study of the association of portal vein thrombosis characteristics with overall mortality (multicenter cohort).
HR: hazard ratio. CI: confidence interval. LT: liver transplant. PVT: portal vein thrombosis. * n = 144. ** n = 61. *** Model: n = 144. P after bootstrapping (1,000 samples); χ 2 11.
DISCUSSION
Key findings
In this study, PVT patients had similar demographic, biochemical, severity of illness, and post-LT outcomes to non-PVT patients (Edmonton cohort). However, within this cohort, complete/occlusive PVT adversely impacted overall survival after adjusting for other covariates reflecting etiology and severity of illness. In a combined cohort of PVT patients from two Canadian transplant centers (Edmonton and London), hepatitis C, complete/occlusive PVT, surgical thrombectomy, and post-transplant portal vein re-occlusion were more frequent in non-surviving PVT patients. On adjusted analysis, only hepatitis C and post-transplant re-occlusion were associated with worse post-LT overall mortality. Furthermore, pre and post-LT anticoagulation did not appear to differentiate between survivors and non-survivors.
Comparisons with the literature
Several clinical factors have been previously demonstrated to be associated with the development of PVT. These include previous interventions for portal hypertension (radiologically or surgically), endoscopic therapy for variceal bleeding, thrombocytopenia, hypoalbuminemia, and advanced liver failure.5 , 6 , 20 - 22 In our initial analysis of PVT vs. non-PVT LT recipients, we could only demonstrate a non-significant trend towards lower albumin amongst PVT patients (p = 0.09).
There is equipoise in the literature regarding the impact of PVT on post-LT outcomes for which our analysis clarifies. Ghabril, et al. reviewed the Organ Procurement and Transplant Network (OPTN) registry between 2002 and 2013 [6.8% (3321/48570) of patients had PVT] and concluded that PVT was independently associated with increased 90-day mortality while PVT at listing was not associated with lower transplant rates, delisting or death.23 ) In contrast, Berry, et al. reported in 2015, using data from UNOS (2002-2013), that amongst 66,506 patients who underwent LT, patients who had PVT at the time of listing (n = 2207) had similar outcomes than those without PVT.24
This difference likely reflects selection bias in the two studies. While general patients with PVT undergoing LT may have similar outcomes to those without PVT, subsets of patients with PVT may have worse outcomes and these may not be ascertained from registry studies as easily as we have demonstrated in the subset of patients with complete/occlusive PVT.23 , 24 ) In our Analysis One, multivariable Cox regression revealed that while the presence of PVT generally did not impact outcome, complete PVT portended a worse outcome.
In our cohort of 144 consecutive LT recipients with PVT (Analysis Two), transplant recipients with PVT who subsequently died were more likely to have complete PVT at LT than survivors (p = 0.02). Furthermore, the use of anticoagulation/antiplatelet therapy either before or after transplant was not significantly different between survivors and non-survivors (p > 0.10 for all comparisons). Similarly, Francoz, et al. showed in a French cohort study that, amongst post-transplant PVT patients with either a patent or partially occluded portal vein, 3-year survival was not different between patients receiving and not receiving anticoagulation.6Additionally, the same authors demonstrated that post-transplant 3-year survival was significantly worse irrespective of anticoagulation status if patients had complete PVT.
Advances in surgical technique have led centers to pushing back the threshold to operate on patients with complex portal vein thrombosis in attempts to establish physiological blood flow. In a study of 174 PVT patients that underwent LT, Hibi, et al. demonstrated that for patients with reestablished physiological portal inflow (n = 149/174), survival was similar to LT recipients without PVT (n = 1,205).10 ) However, patients in whom physiological portal inflow could not be re-established had high rates of rethrombosis and worse long-term survival.10 ) Similarly, in our analysis, post-LT portal vein reocclusion was independently associated with worse outcomes (p = 0.01). While it appears clear that re-establishment of physiological portal inflow is important post-LT, which interventions (e.g. anticoagulation, thrombectomy, grafting, or reconstruction) are better at increasing the chances of achieving such goal are yet to be better studied.25
While this study identified that hepatitis C patients did worse than other etiologies irrespective of PVT status, this was in a cohort of patients that were treated prior to the highly effective new direct antiviral agents in use to treat HCV, therefore this effect will likely not be as significant in the future.26 , 27 ) Nonetheless, we adjusted for this variable to remove its role as a confounder on the impact of PVT on post-LT outcomes.
Previous reports have demonstrated that rates of inherited thrombophilias are higher in cirrhotic patients with PVT than without.4 , 22 Amitrano, et al. have previously reported rates of Factor V Leiden and prothrombin 20210 mutations were present in 13% and 35%, respectively, in a small cohort of 23 cirrhotic patients with PVT.4 ) In a larger cohort of 144 PVT patients who underwent LT, we could only identify 14 (10%) with an inherited thrombophilia. Nevertheless, besides inherited disorders, there are other physiological factors that may predispose advanced cirrhotic patients to PVT including low levels of AD-AMTS13 with high levels of von Willibrand factor, and in some cases hypofibrilolysis.28
While current evidence highlights the risk of thrombosis progression in patients listed for LT, current guidelines do not propose definitive treatment strategies for the management of PVT patients awaiting LT.25 Previous studies have demonstrated that reduced portal vein flow velocity was independently associated with PVT development.29 ) Additionally, patients treated with low molecular weight heparin prior to LT have a portal vein repermeation rate ranging from 46 to 85%.6 , 11 , 30 , 31 Furthermore, the time interval between diagnosis of PVT and initiation of anticoagulation (less than 6 months) may impact the chance of resolution of thrombosis.31 ) A previous systematic review has demonstrated that PVT recurrence was higher in patients who did not undergo preventative strategies after surgery (10.3 vs. 6.2%, respectively).25 Although our data did not demonstrate differences between patients who received anticoagulation and those who did not, post-LT portal vein re-occlusion was independently associated with increased mortality. Hence anticoagulation should nonetheless be considered soon after transplant, unless contraindicated by medical or surgical issues.
Limitations
This study has several limitations that warrant considerations. As a retrospective cohort in nature, it can only comment on associations and not causation. While we had pooled information on all LT recipients with PVT between 2002-2012 at two medium sized Canadian liver transplant centers (Edmonton and London), we only had matching consecutive cohort data of non-PVT LT recipients from one site and hence could only make comparisons based on data from Edmonton (Analysis One). While we admit we did not have complete information for surgical procedures on all 144 patients (e.g. thrombectomy or grafting), we did have complete data on vascular reconstruction for all 144 patients.
Nonetheless, this study builds on the current published literature as our analysis suggests that PVT patients that are well selected with premorbid characteristics may do as well after LT. Furthermore, patients with more complex PVT (complete/occlusive PVT, those requiring thrombectomy, or those who reocclude post-LT) may have worse outcomes and need to be studied further in future studies.
CONCLUSIONS
Well-selected LT patients who had PVT prior to LT had similar post-LT outcomes to controls. Amongst these patients, complete/occlusive PVT and post-LT portal vein re-occlusion were independently associated with worse outcomes. Subgroups of PVT patients with higher risk for post-LT worse outcomes warrant closer evaluation in listing and management peri-LT.
ABBREVIATIONS
CKD: |
chronic kidney disease. |
COPD: |
chronic obstructive pulmonary disease. |
DRI: |
donor risk index. |
HCV: |
Hepatitis C. |
HR: |
Hazard Ratio. |
ICU: |
intensive care unit. |
INR: |
internationalized ratio. |
IQR: |
inter-quartile range. |
LMWH: |
low molecular weight heparin. |
LT: |
liver transplantation. |
MELD: |
Model for End-Stage Liver Disease. |
OTTR: |
Organ Transplant Tracking Record. |
PVT: |
Portal Vein Thrombosis. |
SD: |
standard deviation. |
TP: |
Child Turcotte Pugh. |
UNOS: |
United Network for Organ Sharing. |











 nueva página del texto (beta)
nueva página del texto (beta)