INTRODUCTION
Chronic hepatitis B virus (HBV) infection is one of the major global health issues mainly due to its life-threatening complications including hepatic decompensation, cirrhosis, and hepatocellular carcinoma.1 Worldwide almost 400 million people are affected with HBV, and over 300,000 die every year from HBV-related diseases. Entecavir (ETV), lamivudine (LVD), and tenofovir disoproxil fumarate (TDF) are potent antiviral agents for the treatment of chronic HBV infection.2 Lamivudine, one of the oldest drugs, has been known to cause antiviral resistance (30-70%) and consequent treatment failure; ETV and TDF are associated with less frequent resistance.
TDF, a nucleotide reverse transcriptase inhibitor (NRTI), is a highly effective agent in the treatment of HBV and is considered by some clinicians to be first line antiviral agent due to lack of any documented resistance to the drug and its ability to reverse liver fibrosis.3,4 Although generally it is considered a safe drug and well tolerated, concerns have emerged regarding long-term side effects.5,6 Longterm treatment with TDF has been associated with proximal renal tubular dysfunction and decreased bone mineral density (BMD).2,5 Renal dysfunction has been reported in patients with human immunodeficiency virus (HIV) or chronic HBV receiving TDF.7-9 TDF-induced effects resemble Fanconi syndrome presenting with different manifestations of proximal tubular dysfunction, including metabolic acidosis, glycosuria, hypophosphataemia, and aminoaciduria. Randomized controlled clinical trials have shown that TDF therapy resulted in decreases in BMD as compared with placebo in HIV-infected patients.6,10 In addition, cases of TDF-associated fractures and/or osteomalacia in conjunction with bone metabolic abnormalities have been reported.6,8,11 Possible mechanisms of TDF-induced bone effects include proximal renal tubular dysfunction with resulting hypophosphatemia, abnormalities in Vitamin D metabolism, bone mineralization defects, and alteration in osteoblast gene expression and function.6,12
Fibroblast growth factor 23 (FGF23), a hormone secreted by osteocytes, is involved in the regulation of phosphate metabolism.13,14 In the kidney, FGF23 reduces the expression of sodium phosphate transporters in proximal tubules thereby inducing excessive renal phosphate wasting. FGF23 also inhibits renal 1-hydroxylation of 25OH Vitamin D, leading to reduced 1,25 (OH)2 vitamin D (calcitriol). This in turn reduces the gastrointestinal absorption of phosphate and calcium. The clinical picture of excess FGF23, typified by oncogenic osteomalasia, is characterized by renal phosphate wasting, hypophosphatemia, low serum calcitriol, impaired bone mineralization, and increased risk of fragility fractures.13,14 Recently, we have reported a case demonstrating elevated FGF23 in an HIVinfected man on TDF with elevated FGF23 levels spontaneously declining on withdrawal of TDF and with reversal of phosphate wasting.8
Data are limited on the longer-term effects of TDF on renal phosphate handling, Vitamin D metabolism and BMD in subjects with chronic HBV infection. Thus, we evaluated cross-sectionally, HBV-infected subjects treated for over 1 year with TDF or other antiviral therapy and the correlation with hypophosphatemia, renal phosphate wasting, renal dysfunction and reduced BMD and the association with FGF23 abnormalities. To our knowledge, this is the first clinical study that has specifically explored the role of FGF23 together with other markers of metabolic bone disease in patients with chronic hepatitis B on antiviral agents and in untreated patients.
MATERIAL AND METHODS
This cross-sectional study involved a convenience sample of in consenting chronic HBV-infected men and women being followed at a tertiary urban hospital Outpatient Gastroenterology Clinic in Vancouver, Canada between February 2014 and July 2014. The protocol and informed consent were approved by the institutional Clinical Research Ethics Board of the University of British Columbia.
All subjects were over 19 years, were on treatment for chronic HBV infection for over 1 year with ETV (0.5 mg/ day), LVD (100 mg/day), or TDF (300 mg/day). Control subjects (CON) were recruited from chronic HVB-infected subjects who were not treated with any antiviral medications prior to study enrollment.
Exclusion criteria were as follows: HIV/HCV co-infection, other immunodeficiency state, current bisphosphonate therapy, liver cirrhosis, hepatocellular/other carcinoma, creatinine > 120 umol/L, underlying endocrine disorders known to cause abnormality in phosphate metabolism such as hypo/hyperparathyroidism, or acromegaly.
Demographic data and fracture risk factor (FRAX) data were collected. This included ethnicity, gender, age, smoking, alcohol consumption, exercise, medications, fracture history, family history of hip fracture, arthritis, calcium intake, vitamin D supplementation, substance abuse, HBV duration of infection, and prior HBV therapies. Height and weight were measured.
The primary observations were serum phosphate (PO4) and FGF23; secondary observations included serum calcium (Ca), creatinine (Cr), estimated glomerular filtration rate (eGFR), albumin, bilirubin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), prothrombin time (PT), and HBV-DNA. In subjects with abnormal levels of PO4, Ca, and/or FGF23, serum intact parathyroid hormone (iPTH), 25-hydroxy vitamin D (25-OH Vitamin D), and 24 h urinary excretion of PO4 and Ca were measured.
Routine serum chemistries for PO4, Ca, Cr, eGFR, and liver function tests were assessed by autoanalyzer (Siemens Vista). 25-OH vitamin D and iPTH were measured by automated chemiluminescence immunoassay. Serum FGF23 was measured using a two-site enzyme linked immunosorbent assay (ELISA) with antibodies directed to the carboxy-terminal (C-term) portion of FGF23 (Immutopics, CA). The FGF23 intraand interassay coefficients of variation are 6% and 15%, respectively.
Hepatitis B viral load testing was performed by the provincial laboratory based at the St. Paul’s Hospital Virology Laboratory (Vancouver, BC), using the Roche Taqman Hepatitis B PCR Assay (Roche Diagnostics, Mississauga ON) as part of standard clinical care. The lower detection limit of the assay is 20 IU/mL and undetectable is defined as less than the lower detection limit.
Bone mineral density (BMD) was assessed on subjects with abnormal levels of PO4, Ca, and/or FGF23 subjects with abnormal levels of PO4, Ca, and/or FGF23 by DXA scanning of the spine and hip (Hologic Discovery Waltham MA). FRAX 10-year fracture risk, for a major osteoporetic fracture, was calculated based on clinical factors including subject age (in year), gender, weight, height, previous fracture, previous history of parental hip fracture, corticosteroid glucocorticoid use, a concomitant diagnosis of rheumatoid arthritis, a secondary cause of osteoporosis, current smoking, and alcohol intake > 3 units per day.
Statistical analysis
Considering phosphate normal range and 0.28 effect size, we needed at least 144 subjects (36 in each group) to reach a 0.8 power and 0.05 alpha. For continuous variables, two-sample student t test were used to compare averages among groups. If there were more than 3 groups in comparison, categorical variables were tested using χ2 test or Fisher’s exact test. Continuous variables were reported as mean ± standard deviation, unless otherwise specified. Logistic regression was used to determine factors associated with low phosphate. A P-value of <0.05 was considered statistically significant. Statistical analysis was performed using a statistical software package (SPSS version 21.0, SPSS Inc., Chicago, IL).
RESULTS
Subjects demographics and laboratory data
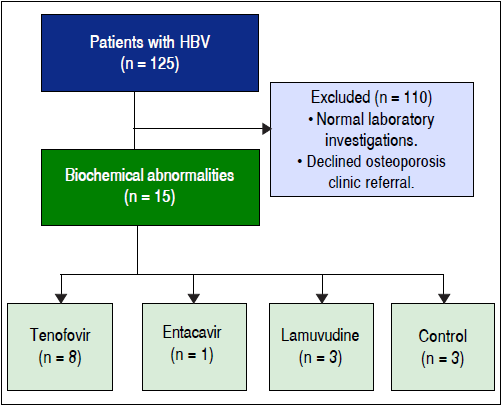
A total of 125 chronic HBV-infected subjects were enrolled in the study as follow: CON (n = 36), TDF (n = 36), ETV (n = 17), and LVD (n = 36) with baseline characteristics similar between groups (Table 1). The mean age was 51 years (range 28 to 77 years). The total duration of therapy ranged 1 to 12 years with mean of 2.8 years for TDF group; 1.5 to 7 years with mean of 3.7 years for ETV group; and 1 to 10.5 years with mean of 3.9 years for LVD group. The patients were mostly men (65%) with majority being Asian (97% of total). The mean baseline serum HBV-DNA level was 5.5 log IU/mL ± 6.4 log IU/mL in the control group and undetectable in the antiviral treatment groups. The mean duration of antiviral therapy was 3.5 years (1-12 years).
Serum PO4, Ca, Cr, eGFR, albumin, total bilirubin, AST, ALT, GGT, and INR were comparable between the 3 treatment groups (Table 2).
Subjects with abnormal levels of phosphate, calcium, and/or FGF23
Table 3 demonstrates laboratory results of subjects with abnormal phosphate metabolism. Out of 125 subjects enrolled 18 subjects (14.4%) had abnormalities in serum calcium, phosphate, or FGF23. The most common abnormality, in 13 subjects (72%) was hypophosphatemia. Of the hypophosphatemic subjects, 5 (38.5%) were on TDF, 4 (30.8%) were on LVD, 1 (7.7%) was on ETV, and 3 (23%) were not on HBV therapy.
Table 3 Clinical features of patients with abnormal serum phosphate, calcium, and/or FGF23.
*Estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease study group formula (MDRD). FGF23: fibroblast growth factor23.
TDF treatment was associated with increased FGF23 in 4 subjects (11.1% of TDF-treated); only one of these 4 subjects had serum phosphate below the lab normal range. No subjects on other HBV therapies had increased FGF23 or hypophosphatemia. One CON subjects had elevated FGF23 without any other laboratory abnormalities. Three subjects had hypocalcemia (with normal FGF23), 2 (5.6%) of whom were on TDF and 1 CON. Our study did not have the power to detect statistical significance in the association between FGF23 abnormalities and TDF therapy.
Association of FGF23 abnormalities with BMD and extended laboratory testing
Among 18 subjects with abnormal phosphate, calcium and/or FGF23, 15 agreed to undergo further investigated for BMD and 13 agreed to be investigated for other biomarkers involved in bone metabolism; 8 on TDF, 3 on LVD, 1 on ETV, and 3 on no therapy; no treated subject stopped treatment at the time of the further investigations (Figure 1). Demographic characteristics and laboratory results of these 15 subjects are summarized in Tables 4 and 5, respectively. The mean age was 45.6 ± 8.1 years. Among 15 subjects, 5 of them were taking multivitamin, one was taking a calcium supplement, and one was taking a vitamin D supplement (Table 4). Two subjects had prior fracture after fall from standing height. BMD, serum chemistries and urine chemistries are summarized in table 5. Serum 25-OH Vitamin D was insufficient (50-75 nmol/L) in 5 subjects (38%) or low (< 50 nmol/L) in 5 subjects (38%) (Table 5). One subject has 1 had elevated iPTH. All tested subjects in this subgroup had normal serum Ca and PO4. Two of 13 subjects with 24 h urine results, had elevated urine calcium and two had increased phosphate excretion. No patient with FGF23 abnormalities had urine abnormalities.
Table 4 Demographic of selected HBV-infected subjects with biochemical abnormalities.
*Chinese, Japanese, Korean.
Table 5 Clinical features of subjects with abnormal serum phosphate, calcium, and/or FGF23.
FGF23: fibroblast growth factor23.
Tables 6 report BMD cohort of selected HBV-infected subjects with abnormal serum level of Ca, Po4, and/or FGF23. Spine and hip BMD was not significantly different between any treatment or CON groups. FRAX 10-year fracture risk of selected HBV-infected subjects with abnormal serum level of Ca, Po4, and/or FGF23 is shown in Table 7. Six (40%) subjects had Z-scores less than predicted for age at the spine or hip of whom one TDF-treated subject had elevated FGF23 (with normal 24 h urine Ca and Po4 excretion). Low or insufficient vitamin D was seen in 3 out of 6 TDF-treated subjects, 1 out of 3 CON subjects and 2 out of LVD/ETV-treated subjects. Spine and hip BMD was not significantly different between any treatment or CON groups. These subjects were at low fracture risk with few clinical risk factors, younger age, and mostly normal BMD. Mean FRAX score was 2.33% (range 1.1-4.1) for major OP fracture and 0.29% (range 0-0.8) for a hip fracture.
Table 6 Bone mineral density in subjects with abnormal serum phosphate, calcium, and/or FGF23.
TDF: tenofovir. Other: lamivdine (n = 3) or entacavir (n = 1). Con: no therapy (n = 3). Data are expressed as Mean ± SD.
DISCUSSION
Our study confirms the frequent occurrence of abnormalities in bone metabolism in subjects with HBV treated with antiviral therapy for over 1 year.
Most frequently, these abnormalities involve vitamin D insufficiency. As shown in table 5, thirteen subjects (out of 15 with abnormal serum calcium, phosphate, and/ or FGF23) had additional work up. Interestingly 77% of them had insufficient/low 25-OH vitamin D. All TDFtreated subjects had low 25-OH vitamin D. There are a number of factors which may contribute to Vitamin D insufficiency in this population. Low frequency of supplementation in a region with low sunlight exposure may result in Vitamin D insufficiency which has been welldocumented in this population.15 Additionally, the vast majority of subjects are Asian who may have impaired Vitamin D skin synthesis attributable to skin pigmentation. Although there were no demonstrated abnormalities in either hepatocellular dysfunction or elevated liver enzymes, it is possible that there is an acquired defect in vitamin D hydroxylation in liver in these patients with chronic HBV although this is less likely given that HBV was adequately suppressed in the antiviral treated patients. Finally, there may be impairment of Vitamin D metabolism either di rectly or indirectly attributable to HBV therapies. Vitamin D insufficiency may have long-term fracture consequences by means of secondary hyperparathyroidism where physiologically-elevated PTH may promote excessive bone resorption and long-term bone loss. Our study sample is too small to demonstrate these effects.
Clinical hypophosphatemia was also observed in our sample, as has been reported elsewhere.5,7-9 In the current study, we showed that 14% of TDF-treated subjects had initially hypophosphatemia. Incidence of hypophosphatemia associated with TDF has been shown to be between 6.5% and 35.5%.16,17 This difference might be due to other factors such as duration of treatment, diet, other medications or duration of disease. In the current study we observed that 11% of LVD-treated subjects and 8% on CON subjects had initially hypophosphatemia. There was no statistically significant difference among our groups. The fact that we observed hypophosphatemia in not-TDF treated subjects raise the possibility of involvement of other factors such as diet, concurrent existence of other diseases, and duration of HBV-infection as the pathogenesis on hypophosphatemia in our subjects.
Although the anticipated increases in FGF23 were not often seen, perhaps increases within the lab normal range could lead to mild abnormalities in serum and urine phosphate which could lead to long term harm to bone. There is also physiologic variability in Ca, PO4, and FGF23 levels which may obscure minor effects in these parameters which may have longer-term consequences. Only 11% of TDF-treated subjects had elevated FGF23 levels and only one subjects out of these four subjects had hypophosphatemia. FGF23 has role in regulation of metabolism of phosphate and vitamin D.14 It enhances renal phosphate excretion and inhibits renal 25-OH-vitamin D hydroxylation. While previously in a case of HIV-infected subject treated with TDF we concluded that FGF23 may be responsible for a component of the phosphate wasting, in the current study we did not see any correlation between TDF and hypophosphatemia in HBV-infected subjects treated with TDF. This discrepancy between current study and our previous study might be due to variable quality in FGF23 ELISA method, co-administration of other antiretroviral medications, use of other concomitant drugs, duration of treatment with TDF, other co-morbidities, degree of tubular dysfunction or other unknown mechanisms. It is also possible that different mechanisms of disease could be seen in different individual subjects.
Among 15 subjects who were further investigated for BMD, 6 (40%) of them had Z-score less than predicted for age-matched control at their hip or spine. There may be differences between the antiviral therapies and their effects on bone metabolism. Though we have limited numbers of patients in each treatment group, our data would suggest that greater bone metabolic effects are seen with TDF than with other antivirals. Consistent with our data, TDF-treatment of both HIV-infected subjects and HBVinfected subjects has been associated with a decline in BMD.6,10-11,18 There may also be a synergy between bone adverse effects of liver disease and the adverse effects of the antivirals used to treat the liver disease. These effects would be impossible to differentiate in our small uncontrolled sample. We did not see any correlation between low BMD and classic risk factors, such as age and smoking. This could be due to small sample size in our study.
We have only a very small sample of subjects with BMD and extended chemistry data. In this cross-sectional sample, we are unable to demonstrate correlations between indicators of bone metabolism and BMD, though in greater numbers of patients these associations may exist. Also, FRAX estimates of fracture risk assume a healthy normal population and may not be applicable to patients either with HBV or HBV therapy or both. FRAX also is relevant to an older population where a 10-year timeframe is reasonable. It should be considered, however, that a younger HBV population on potentially very long term treatment may be at an increased lifetime fracture risk and longer-term outcome studies are needed.
It is also likely that there are patients with responses to HBV antivirals, particularly TDF, who have exaggerated FGF23 and bone adverse effects as have been reported.6,19 For this reason, there should be heightened awareness amongst treating physicians of the potential for Vitamin D, FGF23 and bone metabolism adverse events in HBV subjects on antivirals, particularly TDF. It may be reasonable to suggest supplementation with 2000IU Vitamin D daily in all TDF-treated HBV subjects as a relatively benign means of minimizing potential adverse effects on bone.
We acknowledge the limitations of our study, specifically the small sample size. Larger, controlled studies are required to assess direct meaningful effect of anti-HBV therapy on Vitamin D deficiency and BMD loss. The current study underlines the potential importance of BMD role in subjects with chronic HBV infection who are at risk of a major osteoporotic fracture.
In conclusion, we observed that treatment of HBV-infected subjects with antiviral therapy for more than 1 year was associated with abnormalities in bone metabolism, particularly insufficient/low Vitamin D. We also demonstrated that FGF23 abnormalities alone cannot predict low BMD or bone biochemical abnormalities in patients on anti-HBV therapy. It is possible that more marked FGF23 abnormalities may lead to decreases in BMD with long term increases in fracture risk. This observed abnormality may not be only related to an underlying liver disease but instead may be a result of antiviral therapy.











 text new page (beta)
text new page (beta)