Introduction
Torsion of the hepatic vein causing an early congestion of the liver graft after liver transplantation is rare but is life-threatening.1-3 Recently, we experienced such a patient who received a re-exploration to reduce torsion after detection. Congestion necrosis with geographic calcification of the graft subsequently occurred. Graft calcification has seldom been reported.4 In this case report, we discuss the possible contributing factors.
Case report
A 46-year-old man, a carrier of hepatitis B viral infection, developed liver cirrhosis (Child-class B) and portal hypertension, with hypersplenism and thrombocytopenia. Hepatocellular carcinoma (HCC) was found four months prior to the transplantation surgery. Transcatheter hepatic arterial chemo- embolization (TACE) of HCC was undertaken at that time. However, viable HCC could be demonstrated on the follow-up computed tomographic (CT) scan study. He underwent a living-related donor liver transplantation (LRDLT) with left liver graft (segments II, III and IV, including the middle hepatic vein) from his son on October 16, 2013. Before transplantation, his MELD score was 7.8. The graft was 834 mL and the (GRWR) was 1.04%. The ratio of the remnant liver of his son was 62%. The pathological findings of the liver tumor showed HCC combined with cholangiocarcinoma (Figure 1). Anastomosis was performed as the following: MHV (graft) direct to RHV (recipient); LHV (graft) to the common orifice of MHV and LHV (recipient) using a segment of a cryopreserved iliac vein graft as a bridge; left portal vein (graft) to main portal vein (recipient); LHA (graft) to LHA (recipient); and LHD (graft) to LHD (recipient), respectively. The operation course was smooth. He left the ICU and was transferred to an ordinary ward on the 4th day post-operation. On the 7th day post-operation, jaundice, and fatigue were unexpectedly found. Leukocytosis, as well as elevated AST, ALT and bilirubin levels were noted. An emergency liver tri-phase CT showed heterogeneous enhancement of the liver parenchyma, with delayed opacification of the intrahepatic hepatic venous branches with suboptimal enhancement of the distal LHV. Torsion of LHV with lumen obstruction was suspected (Figure 2). Emergency re-exploration was soon performed. There was an about 10-degree clockwise torsion of the graft with luminal stenosis of the left hepatic vein. Congestion with darkened discoloration of the graft was found. Intraoperatively, the torsion of the left hepatic vein was soon reduced manually. The left hepatic vein was opened for examination, and no thrombus was found. After a check of the patency of the venous lumen by intraoperative echogram, the opening was repaired. Subsequently, pexy of the graft was completed. The pathology of the liver biopsy revealed an extensive necrosis. The patient remained in the ICU for intensive care after the reexploration. The option of re-transplantation had been suggested but the patient refused. To assess the recovery of the graft necrosis and the patency of the vessels, three sessions of follow-up liver triphase CT studies were undertaken on the 3rd, 8th, and 12th weeks after re-exploration. Geographic calcification of the graft with some resolution of necrosis and graft regeneration with an increasing size was subsequently found. However, the patient died of pneumonia and sepsis at the end of the 13th week, post operation.
Discussion
Hepatic vein torsion of the liver graft occurred unexpectedly on the 7th postoperative day in our patient. Venous occlusion (or stenosis) had been reported in literature but torsion or kinking as the cause was not common. 1-3,5-8 Egawa, et al. mentioned outflow obstruction could occur in either early onset or late onset.3 Such lethal complications during LRDLT had been reported.5,6 The torsion in our patient was confirmed by an imaging study and further exploration. We propose the following three possible factors contributing to this rare sequela. Firstly, compared with the whole liver as the graft in cadaveric transplantation, the liver graft in LRDLT is relatively small. The incidence of hepatic venous obstruction increased to 5-13% in LRDLT.1,9 The discrepancy of the size between the graft and the recipient’s right upper guardant cavity occurs.1,2,5 After 0the repositioning of the graft into the total space of the hepatectomy (recipient), some dead space still remains. This affords an opportunity of torsion or a twist of the graft that may subsequently cause a torsion of the vessel, usually, a rotation to the contralateral side. As Inomata et al mentioned,8 torsion, or twist is especially vulnerable to occur when the graft is relatively small, such as left lobe or left lateral segment or less. Our patient’s graft was left lobe of the liver.
Secondly, in LRDLT, the graft usually regenerates in all directions within the limited subphrenic space. A rapid regeneration of the liver graft may push itself toward the ventral side when the graft enlarges.10 The “push” affords a potential risk of the torsion. Some had reported the cause of late onset included fibrosis, parenchymal hypertrophy, and a slow-developing twist by graft growth.3
Thirdly, an inadequate pexy of the graft during the initial transplantation surgery increases the possibility of the torsion. Fixation of the liver graft by suturing the ligament or soft tissues to the recipient’s ligaments or peritoneum may optimize graft orientation in the hepatic fossa and prevent graft rotation. Some surgeons have used a tissue expander inserted into the space adjacent to the graft in order to stabilize the position.11
To minimize the progression of such complications of hepatic venous outflow, early detection is important.1,3 Early correction is the key survival determinant of both the graft and the patient. A high suspicion of vascular problem is mandatory when an unusual manifestation of symptoms or signs or laboratory data was found. To detect the torsion earlier, Doppler ultrasound could be applied to investigate hepatic venous flow of liver grafts.1,3,8 However, some immediate postoperative artifacts may interfere with the ultrasound interpretation. The imaging diagnosis of the graft congestion in our patient was established by the liver triphase CT, as Park’s suggestion.7 Some authors recommended using magnetic resonance imaging (MRI) to define the score of post-transplant congestion of the graft.12

Figure 1 Intraoperative findings of the first and the second sessions of surgery. A. The graft in situ after transplantation (in the initial operation). B. During re-exploration, congestion of the liver graft after torsion of the left hepatic vein was noted (the intraoperative echogram revealed the patient’s blood flow of the hepatic artery and the portal vein.).
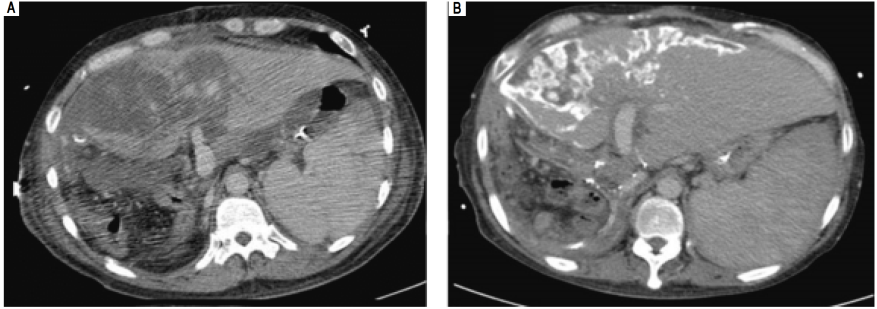
Figure 2 The follow-up liver triphase CT studies after re-exploration. A. CT study of 3rd week: A large portion of hypo-enhancement with heterogeneity of the liver parenchyma in the graft was noted. Congestion with hypo-perfusion was likely. B. CT study of 8th week: The liver graft enlarged in size, compatible with liver regeneration. Geographic calcification was noted in the previous congestion areas of the liver graft, probably resulting from congestion necrosis. Some resolution of necrosis was present.
After detection, surgical reintervention is mandatory for repositioning of the graft or redo of venous anastomosis or retransplantation.1-3,6
Early congestion of the graft is a serious problem, especially when the extent is substantial. Congestion, caused by the venous outflow (hepatic vein) obstruction (either torsion, twisting, or compression), increases the intrahepatic venous pressure and resistance, thus affecting the inflow of both the portal vein and the hepatic artery. Consequently, the supply of nutrients and oxygen is interrupted, increasing an ischemic necrotic progression of the graft.13,14 The insufficient perfusion causes an acute inflammatory response, leading to significant damage, and atrophic change to the corresponding hepatic area, as well as bile duct proliferation, lymphoplasmacytic inflammation, and periportal fibrosis, leading to liver failure and even mortality, especially when the remaining functioning area is too limited to meet metabolic demands.13,14 Congestion necrosis of the liver graft of our patient was confirmed by pathologists from a liver graft biopsy. Retransplantation to treat severe hepatic necrosis of a graft may be the treatment of choice in above mentioned situations.
Calcification of the liver graft had previously rarely been discussed.4 We believe that such phenomena probably are underreported in the transplant literature. Shibuya A found diffuse hepatic calcification as a sequela to shock liver.15 Calcification develops in the degenerative area of the hepatic lobule following parenchymal ischemia after overt shock lasting for 2 days. The microscopic findings may reveal hepatic parenchymal necrosis and tiny calcifications in the central to midzonal area of the lobule. From a rabbit animal model, after extensive hemorrhage, several morphologic changes of the liver occurs, including necrosis, mineralization, severe cell damage which enhanced apoptotic cell engulfment by hepatic phagocytes, with the formation of vacuoles and calcifications.16,17 Ischemic stress induces calcium accumulation also at the cellular level, disturbances of intracellular Ca2+ homestasis, caused by impaired energy metabolism and/or plasmalemmal alterations.17 The elevated intracellular calcium concentration induces cytoskeletal modifications, and alters cell shape. Then, activation of phospholipases causes perpetuation of membrane damage, furthermore, mitochondrial calcification. Subsequently, there are some changes of hepatocytes, including cell swelling, distension of various cellular organelles, clumping with random degradation of nuclear DNA, extensive plasma membrane endocytosis.16,17 An intracellular self-digesting pathway can finally result in cell death. Even in the non-transplant liver, congestion could also cause centrilobular necrosis.13,14 In the congested area, the portal flow may move backward causing the portal vein to become a substitute for the vein drainage. Damage of the hepatic venular wall and sinusoidal outlets occurs following endothelitis, with predominant cell drop-out and sinusoidal congestion, leading to a perivenular fibrosis.
The sequential follow-up CT studies of our patient showed geographic distribution of calcification of the liver graft. We attribute the geographic or uneven distribution somewhat to resolution of the necrosis and somewhat to regeneration of the hepatocytes. Among the cases of calcification, small discrete islands of liver tissues could be noted on CT studies. We propose the cells in these islands may be viable.
Our patient died of sepsis. Infection became the main cause of his death. We propose three possible factors affecting such an outcome.
The first, the graft congestion slows down the flow of the portal vein and the superior mesenteric vein. The resultant congestion of the intestinal mucosa may have allowed the entrance of endotoxins into the liver through the portal vein, leading to portal endotoxaemia.13,18-20
The second, the liver plays a protective role for microorganisms. In acute phase reaction, the liver plays a central role in the clearance of portal blood from microorganisms. A congested graft with insufficient effective volume interferes with this function significantly. The infectious conditions also decrease the inhibitory effect of the proinflammatory cytokines.18 Theoretically, numerous conflicting interactions between inflammation and liver regeneration occur. Thus, a vicious circle occurs, that is, the impaired liver enhances the spreading of bacterial infections, and vice-versa.19 Shibayama, et al. suggested the synergism between liver congestion and endotoxaemia may be a cause of hepatic failure.20
The third, after infection develops, the speed of regeneration of the graft may become limited.18 Furthermore, the septic-related cholestasis based on a cytokine-mediated inhibition of bilirubin excretion may contribute to the increased serum bilirubin. Our patient presented hyperbilirulinemia in the treatment course. The bile stasis of the patient aggravated the liver functions.
How to avoid this torsion complication is important. Two main ways are proposed. The first is to avoid the use of smaller liver grafts. If the discrepancy between the size of the donor’s liver graft and the size of the recipient’s post-hepatectomy fossa is larger, the opportunity of torsion may become higher. The second is to pexy of the liver graft if its size is smaller. Generally speaking, left liver graft is relatively small and rotation occurs more easily. The left liver usually has some remnant falciform ligament or round ligament. To pexy with suturing of these soft tissue onto the residual ligament or soft tissue following hepatectomy of the recipient may avoid or decrease the occurrence of graft torsion.
We conclude that congestion necrosis of the graft after torsion of the hepatic vein is a rare but serious complication after LRLDT. To avoid such a torsion of vessels is necessary and important.











 nueva página del texto (beta)
nueva página del texto (beta)


