Introduction
Minimal hepatic encephalopathy (MHE) is the mildest form of spectrum of HE which is characterized by subtle cognitive and psychomotor deficits in the absence of recognizable clinical symptoms of HE.1 Patients with MHE have increased incidence of motor-vehicle accidents, por quality of life and increased chance of developing episodes of overt hepatic encephalopathy (OHE), which impose a formidable burden on their families and health care system.2-5 Overt episodes of hepatic encephalopathy can occur without warning, and render the patient incapable of self-care, and frequently require hospitalized care.6,7 Overall, MHE is associated with poor prognosis and is probably an independent predictor of survival.8,9
MHE is diagnosed by using neuro-psychometric (NP) tests or neurophysiological tests.10 Treatment of MHE has been mainly targeted towards ammonia lowering therapies which include lactulose,11-16 rifaximin,17,18 probiotics,19-22 L-ornithine-L- aspartate,23-26 L-carnitine,27 branched-chain amino acids,28 etc. Treatment of MHE has been shown to lead to MHE reversal, improve quality of life and also decrease the frequency of occurrence of episodes of OHE.11-26 Therefore, it has been proposed the MHE should be treated.29,30 However, most of the trials have focused only on short-term (8-12 weeks) treatment of MHE. What happens after the treatment of MHE is stopped? Do the patients who once become MHE negative after short-term treatment, remain negative for MHE on follow up? How frequently does MHE relapse on follow up? Does shortterm treatment of MHE decrease the incidence of future episodes of OHE and improve long-term survival? These questions have not been answered by any trial till now.
In this study, we aimed to evaluate the long-term (9 months) efficacy of a short-term (3 month) treatment of MHE with lactulose or rifaximin, for maintenance of remission from MHE, in out-patient cirrhotics diagnosed to have MHE.
Material and methods
This prospective study was conducted at the Gastroenterology out-patient department of a tertiary care institute in northern India. The study consisted of 2 phases:
Consecutive patients with cirrhosis visiting the out-patient department were screened for the presence of MHE. MHE was diagnosed if any two of the five neuro-psychometric tests were positive (details below).
Inclusion criteria were- liver cirrhosis, age 18-65 years and presence of MHE.
Exclusion criteria were- current or recent (< 6 weeks) use of alcohol; use of Lactulose/Lactitol, Probiotics, Metronidazole, Rifaximin within last six weeks; use of interferon therapy, psychoactive drugs, anti-epileptics within last six weeks; infection or gastrointestinal hemorrhage within last six weeks; opium addiction; presence of hepatocellular carcinoma (HCC); history of portosystemic shunt surgery and past history of OHE.
Laboratory assessment was carried out at baseline, after 3 months of treatment and at 6 months of post-treatment follow up. This included hemogram, liver and renal function tests, prothrombin time index, and fasting blood sugar. Child-Turcotte-Pugh (CTP) score and Model for End Stage Liver disease (MELD) score for stage of cirrosis were calculated.
Written informed consent was taken from each patient. The Institutional ethics committee approved the study protocol, and it conformed to ethical guidelines of the 1975 Declaration of Helsinki.
Diagnosis of minimal hepatic encephalopathy
Patients with a Mini Mental State Examination score > 25 were considered for neuropsychometric testing using number connection test A (NCT-A), figure connection test-A (FCT-A), digit symbol test (DST), picture completion test (PCT), and block design test (BDT).1,31,32 Diagnosis of MHE was made if two or more neuropsychometric tests were impaired beyond two standard deviations of known control values.1 Neuropsychometric test results were expressed as Z-scores, indicating differences (in standard deviation) between observed and expected scores. A Z-score of < -2 indicated that the result was impaired beyond 2 standard deviations of the known control value. The mean Z-score was calculated for each patient in order to avoid bias related to multiple comparisons. Mean Z-score (Δ mZS) after three months of treatment served as a quantitative measure of psychometric change.
Treatment phase (3 months)
Patients with MHE were randomized into two groups (group A and B) using computer-generated randomization (1:1) and sealed opaque envelope method. Group A patients to receive Lactulose (Duphalac; Abbott India Limited, Mumbai, India) per orally, 30-120 mL/day and group B patients to receive Rifaximin tablets (Rifagut; Sun Pharmaceutical Industries Ltd, Mumbai, India) per orally, 400mg three times/day, for three months. Dosage of lactulose was adjusted to make the patient pass 2 to 3 semi-formed stools. Patients were followed up at regular intervals for assessment of compliance and for the development of any new symptoms/treatment related adverse events. At the end of 3 months, repeat neuropsychometric testing was done; specific therapy for MHE (Lactulose/ Rifaximin) was stopped while standard medical therapy for cirrhosis was continued.
Follow-up phase (6 months)
All patients were followed up after six months of stopping treatment (i.e., 9 months post-randomization), the general condition of each patient was enquired telephonically. Patients who could not be contacted were labeled as ‘lost to follow-up’. Patients who had not suffered from any adverse events like OHE or death in the 6 month followup period were called to the hospital for repeat evaluation. Routine investigations and testing for MHE was performed in these patients.
Diet and concurrent therapy
During the entire study period, patients were allowed to take a normal protein diet (~1 g/kg body weight, predominantly vegetable/casein based). Salt restriction was advised to those who had ascites. Standard treatment for cirrhosis including diuretics and β -blockers were given, if indicated.
Statistical analysis
Data are presented as means with 95% confidence intervals (CI) for quantitative variables and as proportions with 95% CI for qualitative variables. The changes in NP test results and within and between the groups were assessed by analysis of variance. Multiple logistic regression analysis was performed to assess the factors associatedwith MHE relapse, and results expressed as odds ratio (OR) with 95% CI. Fisher’s exact test was performed to assess improvement in MHE on an intention-to-treat basis. A P-value of < 0.05 was considered statistically significant. Statistical analysis was carried out with SPSS software, version 11.5 (SPSS, Chicago, IL).
Results
A total of 527 patients with liver cirrhosis were screened. Out of these, 176 (33.4%) patients were excluded due to various reasons (127 patients had history of recent OHE and were using Lactulose, L-ornithine-L-aspartate or Rifaximin, 44 had history of opium addiction, 27 had history of current/recent (< 6 weeks) use of alcohol, 11 had advanced cardiopulmonary disease, nine had used antibiotics within last six weeks, seven had hepatocellular carcinoma, five were using psychoactive drugs/anti-epileptics, seven had history of gastrointestinal hemorrhage within last six weeks, four had renal insufficiency, and four were on Interferon therapy). A total of 69 patients were excluded due to multiple reasons. The remaining 351 patients were found eligible and tested for MHE. Out of these, 112 (31.9%) patients were diagnosed to have MHE (mean age 55.3 years; 75% males). They were randomized to receive Rifaximin (n = 57; 1200 mg/day) or Lactulose (n = 55; 30-120 ml/day) for three months. Later, these patients were followed up to 9 months (Figure 1). Baseline characteristics of the 2 groups were similar (Table 1).
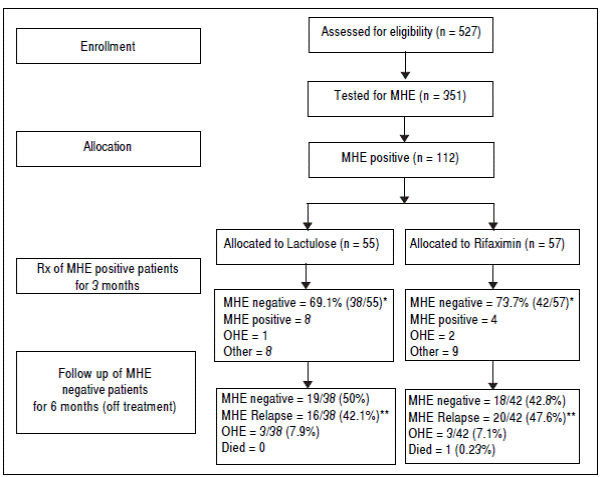
Figure 1 Flow of participants in the study. MHE: minimal hepatic encephalopathy. OHE: overt hepatic encephalopathy. * P value = 0.677. ** P value = 0.274.

Data are expressed as mean (95% confidence interval) or number (percent). P value is non-significant for all comparison between both the arms. CTP: Child Turcotte Pugh. MELD: Model for End Stage Liver Disease. NAFLD: Non Alcoholic Fatty Liver Disease.
Table 1 Baseline characteristics of patients.
Treatment phase (3 months)
The detailed results of treatment phase are available elsewhere.33 In brief, at two weeks, 30/57 patients (52.6%) in the Rifaximin group experienced MHE reversal compared to 22/55 (40.0%) in the Lactulose group (based on the intention-to-treat population). The absolute difference in percentages with MHE reversal was calculated to be 12.6% (95% C.I. -2.8% to 27.2%).
At 3 months, forty-two out of 57 patients (73.7%) in the Rifaximin group experienced MHE reversal compared to 38 out of 55 (69.1%) in the Lactulose group (based on the intention-to-treat population) (p = 0.677). The absolute difference in percentages with reversal (Rifaximin-Lactulose) was calculated to be 4.6% (95% C.I.-9.3% to 18.4%). This corresponds to an odds ratio of 1.25 (95% C.I. 0.63 to 2.50) for Rifaximin relative to Lactulose.
In the Rifaximin group, two patients developed OHE, one patient went for liver transplant and eight were lost to follow up. In the Lactulose group, one patient developed OHE, two patients developed hepatocellular carcinoma, two patients died, and 4 were lost to follow up.
Follow-up phase (6 months)
In the Rifaximin group, at 6 months follow-up, 18 (42.8%) out of 42 patients (who had become MHE negative with treatment) were still MHE negative. Out of the rest, 20 patients (47.6%) had relapse of MHE, while 3 (7.1%) developed OHE and one (0.23%) expired. Out of the 4 patients who had persisted to be MHE positive at 3 months despite treatment, 2 expired (50%), one (25%) developed OHE and one was lost to follow up.
In the lactulose group, at 6 months follow-up, 19 (50%) out of 38 patients (who had become MHE negative with treatment) were still MHE negative. Out of the rest, 16 patients (42.1%) had relapse of MHE and 3 (7.9%) developed OHE. Out of the 8 patients who had persisted to be MHE positive at 3 months despite treatment, 3 (37.5%) developed OHE, 3 expired (37.5%) and 2 were lost to follow up.
Results of individual NP test scores, CTP score and MELD score in the two groups are shown in table 2. Comparison between the 2 groups revealed that the incidence of MHE relapse at 6 months was not significantly different between the Rifaximin and lactulose groups (47.6% 42.1% respectively, p = 0.274). The mean CTP score (8.2 ± 2.3 vs. 6.3 ± 1.9; p = 0.0001) and MELD scores (11.8 ± 4.5 vs. 8.7 ± 3.9; p = 0.0016) of patients who had MHE relapse were higher compared to those who didn’t have a relapse of MHE.

Data are expressed as mean (95% confidence interval).
Table 2 Comparison of psychometric test performance and liver performance status of all patients at baseline, 3 months and 9 months.
The 6-month incidence of development of OHE among patients who became MHE negative at 3 months was not significantly different between the 2 groups (7.1% vs. 7.9% respectively; p = 0.924). However, the incidence of OHE among those who had persisted to be MHE positive at 3 months was significantly higher (25% and 37.5% in the two groups respectively; p = 0.011).
The mortality rate at 6-month follow-up among patients who became MHE negative at 3 months was not significantly different in the 2 groups (0.23% vs. 0% respectively). The 6-month mortality rate among patients who persisted to be MHE positive at 3 months was much higher (50% and 37.5% in the two groups respectively; p = 0.014).
Factors affecting MHE relapse
Various factors like age, sex, CTP score, MELD score, serum bilirubin, platelet count and baseline NP test score (Z-score) were evaluated for association with MHE relapse (Table 3). On univariate analysis, CTP, score, MELD score and Z-score were associated with MHE relapse. On multivariate regression analysis, MELD score was an independent predictor of MHE relapse.
Discussion
The prevalence of MHE in patients with cirrhosis varies between 30% and 84%.1,9,10,34 With treatment, MHE reversal has been reported to occur in about 54-75% of the patients.12,13,17,33 In addition, treatment has been shown to improve health related quality of life, decrease road-traffic accidents, improve daily performance and also decrease the occurrence of episodes of OHE, during the treatment period.3,13,17,18,23 The present study is the first study that followed up patients of MHE after short-term treatment with lactulose or Rifaximin, and found that although about 65% of patients had MHE reversal after treatment for 3 months, almost half of these patients had relapse of MHE at 6 months follow-up. The frequency of MHE relapse, OHE development and mortality were similar in the lactulose or Rifaximin groups. However, the incidence of OHE and mortality were higher among patients who didn’t experience MHE reversal at 3 months compared to those who had initial MHE reversal.
MHE, although common, is not routinely tested in clinical practice, as there are no clinical guidelines recommending the universal screening of MHE.35-37 However, testing of MHE in cirrhotic patients has gained importance in the recent years as it can provide useful and meaningful information at the individual level and help toplan a tailored therapy.35 Some subgroups of patients would benefit the most from testing for MHE as specific recommendations can be made if they have MHE.10,30 For example, active drivers should be cautioned regarding the increased probability of road traffic accidents due to their increased reaction time, and workers engaged in high risk jobs should be counseled against handling of complex/dangerous machinery. In addition, patients who have cognitive impairment and decline in work performance perceived by themselves, relatives or by colleagues should be assessed and, if possible, changes in daily/ working activities should be carried out.30,35 The importance of testing MHE has further been strengthened by a recent study by Ampuero, et al., which showed that MHE is associated with a reduced 5-year survival rate of patients with cirrhosis. Evaluation of MHE could help predict survival times and outcomes of patients with specific MELD scores.38
The decision to initiate medical therapy for MHE ought to be individualized, based on the estimation of the extent of patient’s daily life impairment that can ameliorate with therapy.30,35 In the recent years, various medical therapies that have been found effective for treating MHE patients include lactulose,11-16 rifaximin,17,18 probiotics,19-22 L-ornithine- L- aspartate23-26 and improved nutritional status.39 However, none of these studies have reported the follow up data of the patients after withdrawal of specific therapy for MHE. Thus, the long-term benefit of these therapeutic options for MHE had remained questionable till now. The present study attempted to fill this lacuna in the knowledge regarding the long-term efficacy of treatment of MHE. This study differs from previous studies in that it evaluated the long-term beneficial effect of a short-term therapy with Rifaximin and lactulose, rather than their short-term benefits only. In our study, after treatment with lactulose or Rifaximin for 3 months, about 65% of patients had MHE reversal. These results are similar to the previously reported data on MHE reversal after short-term treatment with lactulose or Rifaximin.13,14,17,18 However, after 6 months of post-treatment follow up, only about 50% of these patients in our study remained negative for MHE, while the rest suffered from adverse outcomes (MHE, OHE or mortality). In a study by Das, et al.5 describing the natural history of MHE, 32 patients who were negative for MHE were followed up for a mean of 5.4 months. At the end of follow up, 68.8% patients were still negative for MHE while the rest had adverse outcomes (MHE in 18.7%, OHE in 6.2% and mortality in 6.2%). These data indicate that the natural history of MHE in patients who become MHE negative with shortterm treatment is worse than those who are MHE negative at the baseline. This is because of the high relapse rate of MHE after as short-term treatment. This suggests that the therapy of MHE may need to be given for a longer period for secondary prevention of MHE.
The prognostic significance of MHE has been reported in another study by Dhiman, et al.40 In this study, the2-year mortality rate among untreated MHE positive patients was significantly higher compared to MHE negative patients (39.1% vs. 22.9% respectively). Similar results were observed in our study, where the 6 month mortality among patients who persisted to be MHE positive at 3 months despite treatment was significantly higher compared to those who became MHE negative (37.5% vs. 0.23% respectively). Also, in concordance with the Dhiman, et al. study, the incidence of OHE was significantly higher among MHE positive patients compared to MHE negative patients in our study (37.5% vs. 8%). MHE with poor psychometric hepatic encephalopathy score was an independent predictor of survival in their study. In a recent study, Patidar, et al.41 have shown that covert HE was associated with worsened survival, increased risk of hospitalization and overt HE development after controlling for the MELD score. These data highlight the need to treat MHE to reduce long-term morbidity and mortality.
The next question arises: How long should patients be treated for secondary prevention of MHE? Although the long-term data for secondary prevention of MHE is not available, similar data for secondary prevention of OHE with ammonia lowering therapies for after treatment of acute episode has been addressed by various studies. In a single-center, open-label study of 120 patients, lactulose therapy was found to be more effective than no active treatment in the prevention of overt hepatic encephalopathy. 42 Later, Bass, et al. showed that the addition of Rifaximin to lactulose reduced the risk of a breakthrough episode of OHE during a 6-month period among patients in remission who had a recent history of recurrent overt hepatic encephalopathy (≥ 2 episodes within the previous 6 months) before enrollment.43 Recently, Neff, et al. has reported that Rifaximin use for greater than 6 months proved to be effective in the management of HE, especially in patients with MELD less than equal to 20.44 As the underlying pathophysiology of MHE and OHE are same, it is understandable that both of these conditions need long term treatment for their secondary prevention. As discussed earlier, specific sub-groups of MHE patients are likely to benefit the most from treatment. Hence, the decision to treat MHE for short-term or long term needs to be individualized.
To conclude, this is the first study to provide the followup data of MHE patients after short-term treatment. Of the patients who became MHE negative after short-term (3 months) treatment with rifaximin/lactulose, almost 50% had a relapse of MHE at 6 months follow-up.
Future multi-centric placebo-controlled studies must address questions regarding the efficacy, safety and costeffectiveness of long-term treatment of MHE with lactulose or rifaximin.











 nova página do texto(beta)
nova página do texto(beta)



