Introduction
Hepatitis B virus (HBV) infection is a serious public health problem. An estimated 240 million people are chronically infected with HBV, and approximately 650,000 individuals die annually from HBV-related cirrhosis and cancer. HBV infection chronicity following an acute infection occurs mainly at birth (90%) or before 5 years old (20-60%) but is rare in adulthood (< 5%).1,2 Therefore, HBV vaccination is the most effective means to prevent HBV infection and to decrease HBV-related morbidity and mortality.3
Korea once was an endemic area for HBV infection; the HBsAg positivity rate was 2.98% in 2010. The consecutive successful HBV vaccination programs conducted by the Korean government, i.e., the Newborn Vaccination since 1995 and the Perinatal Transmission Prevention since 2002, were regarded as the most important factors that reduced seropositivity in children and adolescents from 4-5% to 0.2% in Korea during the past few decades.4-6
HBV vaccination is conducted in accordance with a 0, 1, and 6 month schedule, and the first dose is recommended to be given within 24 h after birth.7,8 A non-responder (NR) is defined as a person with an anti-HBs antibody (Ab) titer < 10 mIU/mL 1-6 months after the 3 dose regimen. Among properly vaccinated individuals, 90% of adults and 95% of children/adolescents show proper immunity (anti-HBs Ab ≥ 10 mIU/mL). Although Korea is now classified as an intermediate HBV endemic area (2-7% prevalence) as a result of the vaccination program, NR status even after proper vaccination is a concern.9,10
Host factors that can contribute to ineffective vaccination include intrauterine infection, a high viral load in the maternal blood, vaccine escape mutants, viral reactivation, host genetic factors, a compromised immune system, and premature birth. Other causative factors include improper storage and transportation of vaccines, and inappropriate timing, interval, or site of vaccination.11,12 The immune response to viral vaccines is influenced by various immunoregulatory genes. Among these genetic determinants, HLA genes are of great interest. The association of HBV vaccine responsiveness and HLA alleles in healthy Korean infants and children was shown in a previous study.13 Cytokines play essential roles in regulating antigen presentation in both innate and adaptive immune responses.14 Associations of cytokine genes and cytokine receptor genes with immune responses to mumps, influenza, and HBV vaccination have been reported.15-17 Recently, an increasing number of studies have shown a correlation between immune responses to the HBV vaccine and the SNPs of immunoregulatory cytokine genes, such as IL-1β, IL-4, IL-10, IL-12B, and IL-13.17-19
IL-4 is a typical pleiotropic T helper (Th) 2 cytokine that plays an important role in humoral and cell-mediated immunity.20IL-4 induces the expression of class II MHC molecules on resting B cells and induces the secretion and expression of IgE and IgG1. IL-4 gene mutations may alter its expression and downstream signaling, which may affect vaccine responses.21IL-12 is an important cytokine that maintains a sufficient number of memory and effector Th1 cells that generate long term protection toward intracellular pathogens.
The aim of the present study was to identify IL-4 and IL-12B gene polymorphisms associated with non-responsiveness to HBV vaccination in a Korean population.
Material and methods
Subjects
Seoul Metropolitan Public Cord Blood Bank provides several laboratory tests, including HBsAg and anti-HBs, for cord blood (CB) donors when they visit the Boramae Medical Center at the age of 9-15 months. During this time, most infants are examined for their response to three doses of the HBV vaccine. Among the 1,737 infants who participated in the donor reward program during the 5 year period (Feb 2007-Jan 2012), 300 infants between the ages of 9-15 month old whose parents were of Korean descent were enrolled. Originally, 8.1% of the total infants of the donor reward program were in the NR group. How- ever, because our intention was to enroll a larger proportion of subjects in the NR group than the epidemiologic percentage, the NR group was increased to include 20% of the total subjects. Cord blood units (CBUs) from the study subjects had been voluntarily donated with informed consent and properly preserved. A proper CBU is defined as follows: a sufficient number of nucleated cells, negative for all serologic tests of infectious diseases, including HBsAg in both CB and maternal blood, negative for potential hazardous medical conditions such as genetic disorders or pregnancy complications, no smoke or alcohol ingestion during pregnancy, full term delivery, and no significant abnormalities or serious diseases at the recontact 6 months after CB donation. The data indicated that all of the study subjects were normal, healthy Korean infants that were delivered full term and vaccinated according to the suggested schedule.
All of the subjects were HBV vaccinated following the recommended protocol. The vaccine (10 μg in 0.5 mL) was intramuscularly injected at 0, 1, and 6 months, and 4 types of recombinant HBV vaccine (Hepavax-gene TF®, Hepamune®, Heptis-BII® and Euvax®) were used. The composition of 4 vaccines was identical with 20 µg/mL of purified hepatitis B surface antigen protein and 0.5 mg of aluminum hydroxide gel adjuvant. The anti-HBs Ab titer was analyzed at least 2 months after the 3rd vaccination.8
We excluded individuals who had asthma, severe atopy, or a history of severe infectious disease or major surgery based on medical records and self-provided information. The design and protocol of this study, including consents, were reviewed and approved by the Institutional Review Board of the Seoul National University Boramae Medical Center (26-2015-103).
Cytokine gene analysis
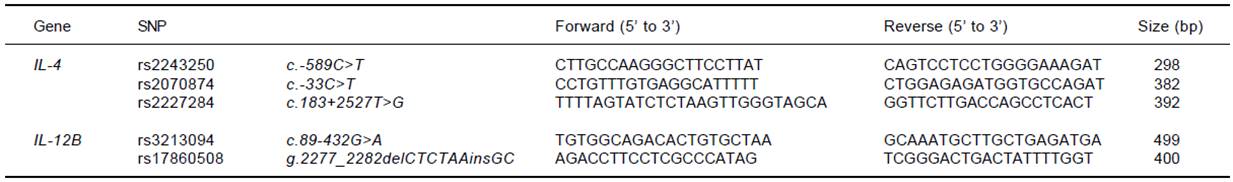
Three SNP sites for the IL-4 gene (rs2243250, rs2070874, and rs2227284) and 2 SNP sites for the IL-12B gene (rs3213094 and rs17860508) were analyzed. Genomic DNA had been extracted from anticoagulated CB using a QIAamp® DNA Mini Kit (Qiagen GmbH, Hilden, Germany) at the time of donation and was cryopreserved at - 80°C until analysis. PCR amplification was performed with sequence-specific primers (Table 1) and h-Taq (Solgent Co., Ltd., Daejeon, Korea) according to the following protocol: 94°C for 5 min, 94°C for 30 s, 58°C for 30 s, and 72°C for 50 s for 35 cycles, followed by 72°C for 10 min. Using a Millipore MSNU030 filter plate (Millipore SAS, Molsheim, France), the purified PCR products were Sanger-sequenced using a BigDye terminator v3.1 sequencing kit and a 3730xl automated sequencer (Applied Biosystems, Foster City, CA).
HBsAg assay & anti-HBs antibody assay
The serum samples were assessed for qualitative HBsAg and quantitative anti-HBs Abs (Architect, Abbott Laboratories, North Chicago, IL) using a chemiluminescent microparticle immunoassay (CMIA) in the clinical laboratory of the Boramae Medical Center. This center was accredited by the Korean Association of Quality Assurance of Clinical Laboratory (KAQACL).
Statistical analysis
SNP frequencies (AFs) were calculated for five cytokine genes by direct counting. The χ2 test or Fisher’s exact test (Fisher’s exact test was used if one of the expected values in 2 x 2 comparison is < 5) was used for AF comparisons. The odds ratios (ORs) and 95% confidence intervals (CIs) were used for 2 x 2 comparisons, showing significant p-values (< 0.05). The interval-by-interval correlation coefficients (Pearson’s R) were used for the linear-by linear-associations. To analyze the contribution of independent SNP factors to the vaccine response, we performed the χ2 test or Fisher’s exact test after stratification by the presence or absence of each factor.22 Where indicated as corrected p (pc ), a Bonferroni correction was applied by multiplying the probability value by the number of comparisons made (e.g, number of alleles compared: 13 for HLA-DRB1 locus) to control for overall type I error.23
Results
Demographic data
A total of 300 infants (153 females and 147 males) were enrolled. All of the subjects were HBV vaccinated on schedule and received the 3rd vaccine more than 2 months (range, 2.7-9.3 months) before anti-HBs quantification. The gestational age and birth weight at birth were 39.7 ± 1.1 weeks and 3.39 ± 0.37 kg, respectively. The age of the infants was 11.3 ± 1.4 months at anti-HBs quantification. Among total subjects, 29% (87/300) were analyzed to determine their antibody titer more than 6 months after the 3rd dose of HBV vaccine.
Anti-HBs antibody titer
We divided the subjects into three subgroups according to the Ab titer: < 10 mIU/mL (non-responder, NR), 10-100 mIU/mL (low-titer responder, LR), and ≥ 100 mIU/ mL (high-titer responder, HR). Among the 300 subjects, 20.3% (61/300), 37.7% (113/300) and 42.0% (126/300) were in the NR, LR, and HR groups, respectively. We adjusted the proportion of each group, which resulted in a higher percentage of NR individuals than the epidemiologic range of 5-10% (our previous result was 8.1%),13 but the subject selection within each group was random. No difference in Ab titer was found between female and male (233.7 and 258.7 mIU/mL) subjects. All of the subjects were negative for HBsAg.
Cytokine gene SNPs associated with anti-HBs antibody titer
We divided the subjects into two groups according to the time interval from the 3rd dose of HBV vaccination to Ab quantification: > 6 months from the 3rd dose and ≤ 6 months from the 3rd dose. We compared anti-HBs Ab titers with the response between the groups. Additionally, we investigated cytokine SNP associations within the ≤ 6 months group. The Ab titer was significantly higher in the ≤ 6 months group than the > 6 months group (276.9 vs. 170.1 mIU/mL, p = 0.006). The NR rate was not different between the groups, but subjects with a high Ab titer (HR) had a slightly increased frequency of NR in the ≤ 6 months group compared to the > 6 months group (45.5 vs. 33.3%, p < 0.1). Among the three Ab titer subgroups (NR, LR, and HR), a lower frequency of NR and a higher frequency of HR were observed in the ≤ 6 months group (p = 0.047) (Table 2).
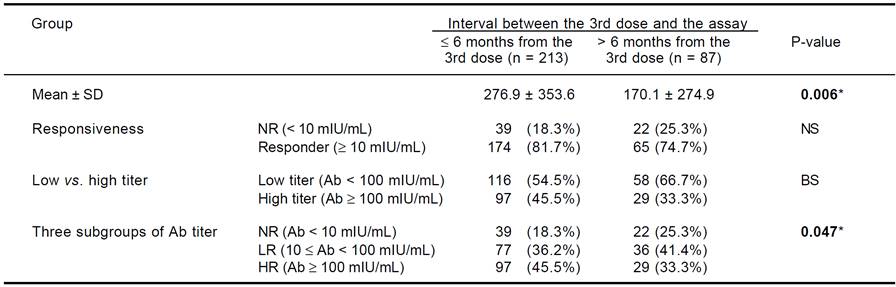
NS: not significant. BS: borderline significant (0.05 ≤ P < 0.1). * Significant association with P < 0.05.
Table 2 Anti-HBs antibody titers according to the interval between the 3rd dose and the antibody assay.
In the comparison of the NR group with the responders, neither the SNP phenotype (individual possessing a given allele) nor the genotype showed an association with non-responsiveness. In the comparison of low titer subjects (NR and LR, Ab < 100 mIU/mL) with the HR group, the rs2243250C allele showed a significantly higher frequency in the latter (p = 0.028, OR = 1.71), and the rs2070874C allele showed a borderline association (0.05 ≤ p < 0.1). In the ≤ 6 months group, the rs2243250C and rs2227284G alleles showed significantly higher frequencies in the HR than the non-HR group (p = 0.014, OR = 2.04 and p = 0.018, OR = 2.25, respectively), and the rs2070874C allele showed a borderline association (0.05 ≤ p <0.1). We also compared SNP distributions among the three Ab titer subgroups (NR, LR, and HR). Although no SNP alleles differed significantly in frequency among the three subgroups of the total subjects, the rs2243250C and rs2227284G alleles showed significantly higher frequencies in HR subjects (p = 0.04 and 0.038, respectively) within the ≤ 6 months group. The allelic frequencies of each SNP were not associated with the response to HBV vaccination (Table 3). The > 6 months group did not show an association between SNPs and the Ab response. No association of IL-12B SNPs with the HBV vaccination response was detected in any comparison.
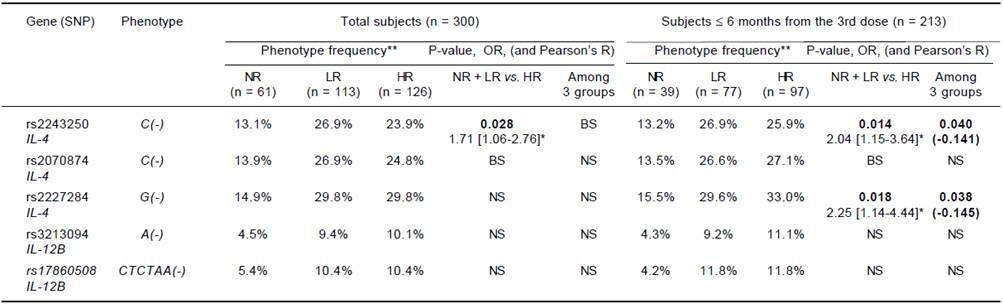
NS: not significant. BS: borderline significant (0.05 ≤ P < 0.1). No cytokine SNPs differed in frequency between NR and responder groups. Significant results (P < 0.05) are listed with P-value and odds ratio [95% confidence interval]* for the low Ab titer and with p-value and interval-by-interval correlation coefficient (Pearson’s R) in parentheses for the linear-by-linear associations. Bold: significant association with P < 0.05. **Frequencies of individuals possessing a given SNP.
Table 3 Association of IL-4 and IL-12B phenotype frequency with responsiveness to HBV vaccination.
For the genotype association, only the rs2243250 genotype (CC, CT and TT) showed a significant association with the Ab titer (p = 0.049) among the total subjects. The rs2243250 and rs2227284 genotypes showed weak dose effects (0.1 < |R| < 0.3) with significant p-values (0.017; 0.015 and 0.028) in the ≤ 6 months subjects, but neither IL-4 in the > 6 months group nor the IL-12B genotype showed dose effects (Table 4).
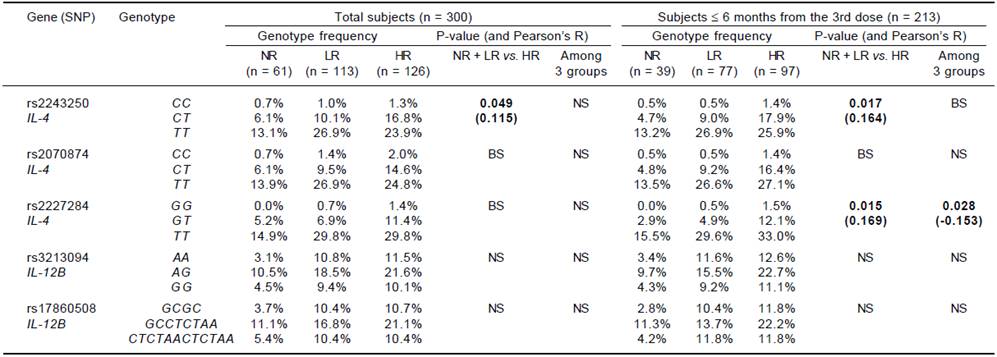
NS: not significant. BS: borderline significant (0.05 ≤ P < 0.1). No cytokine SNP genotype showed a dose effect on Ab titer between NR and responder groups. Significant results (P < 0.05) are listed with p-values and interval-by-interval correlation coefficients (Pearson’s R) in parentheses for the linear-by linear-associations. Bold: significant association with P < 0.05.
Table 4 Association of IL-4 and IL-12B genotypes with responsiveness to HBV vaccination (dose effect of alleles on Ab titer).
Individual risk factors of low Ab titers, rs2243250C (-) and rs2227284G (-), did not show independent associations after stratification with one another but did show a significant combined association within the ≤ 6 months group (OR = 2.44). In addition, the cytokine gene factors (rs2243250C negativity and rs2227284G negativity) and the previously demonstrated factor DRB1*07 showed independent and additive associations with poor responsiveness to HBV vaccination. The rs2243250C genotype showed an association independent of DRB1*07 (OR = 2.11, p = 0.005). However, in the ≤ 6 months subjects, rs2243250C (-) and rs2227284G (-) (OR = 2.36, p = 0.007, pc = 0.044 and OR = 2.55, p = 0.015, respectively) showed associations independent of DRB1*07 (Table 5).
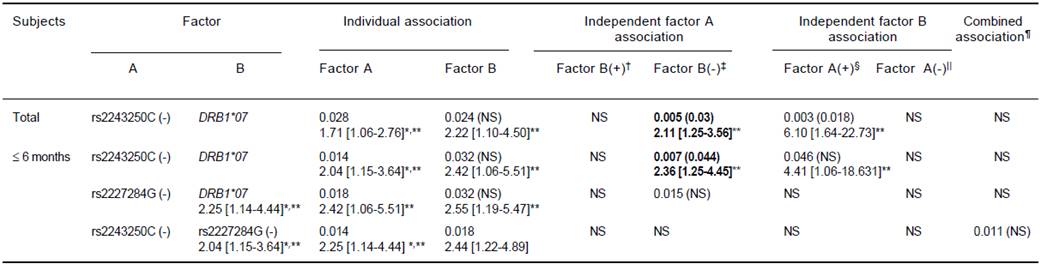
In each cell, the result was obtained from the comparison of low titer (Ab < 100 mIU/mL) vs. high titer groups (Ab ≤ 100 mIU/mL). The first line shows the significant P-value (< 0.05) and the corrected P-value in parenthesis (pc = P x number of comparison) and the second line shows the odds ratio with 95% confidence intervals. ** Bold: significant association after Bonferroni correction (pc < 0.05), normal font: significant only for P. * Individual association factors of low Ab titer using the same statistics as Table 3. † Factor A (+) B (+) vs. Factor A (-) B (+), ‡ Factor A (+) B (-) vs. Factor A (-) B (-).§ Factor A (+) B (+) vs. Factor A (+) B (-), || Factor A (-) B (+) vs. Factor A (-) B (-), ¶Factor A (+) B (+) vs. Factor A (-) B (-); pc = (Px 6) for independent association; pc = (P x 9) for combined association of A and B.22 pc = (P x 13) for individual associations of DRB1.
Table 5 Additive and independent effects of each cytokine gene and HLA vs. cytokine genes on poor response (low antibody titer) to HBV vaccination as represented by odds ratios and P (pc-)-values.
Discussion
HBV infection remains a public health problem with high mortality (22.5/100,000 people) and a considerable socioeconomic burden of KRW 5,453 billion (USD 5 billion), which is approaching 6% of the national budget for health service in Korea.25 Although the successful vaccination program implemented by the Korean government reduced the HBsAg positivity rate, the existence of NR individuals who lack immunity to HBV after proper vaccination is concerning. The factors that influence the immune response to HBV vaccination must be elucidated to overcome the problem of poor responses. Th1 cytokines play a role in the immune response to HBV vaccination. However, no reports have examined HBV vaccine responses and polymorphisms of cytokine genes in a Korean population.
In this study, we examined the IL-4 and IL-12B genes, which may be associated with the response to HBV vaccination. Among the five SNP sites of both cytokine genes, IL-4 gene SNPs (rs2243250 and rs2227284) showed consistently strong associations with low Ab titers. These alleles also showed significant dose effects on anti-HBs Ab titers. These findings are consistent with the previous hypothesis that variability in the IL-4 gene may alter an individual’s response to the HBV vaccine. These polymorphisms may be useful as biomarkers in predicting an individual’s response to different vaccines.18
To provide more comprehensive understanding than an individual’s allele analysis, we analyzed the independent and additive effects of each allele combination. The association of the individual factors, rs2243250C (-) and rs2227284G (-), with ineffective response disappeared after stratification by each allele, which could be the result of a strong positive linkage disequilibrium of the ‘rs2243250C (-) rs2227284G (-)’ haplotypes. When combined, rs2243250C (-) and rs2227284G (-) showed ORs (2.44) similar to those of the individual alleles (2.04 and 2.25). These data suggest a minimal synergistic effect of these alleles on poor responsiveness to HBV vaccination. Diminished secretion of both Th1 (IFN-γ) and Th2 (IL-4 and IL-10) cytokines in HBsAg-stimulated peripheral blood mononuclear cells (PBMCs) from HLA-DR7+ individuals vaccinated for HBV suggests that the expression of HLA-DR7 may influence cytokine secretion by HBsAg-specific T cells after HBV vaccination,26 and the DRB1*07 allele was a strong and independent risk factor for poor responsiveness to vaccination in our previous study.13 Therefore, we analyzed the independent and additive effect of DRB1*07 with the above factors on vaccine response. The independent association of rs2243250C (-) and rs2227284G (-) with poor responsiveness after stratification by DRB1*07 indicates that these cytokine genes play a role in the Ab response after HBV vaccination, particularly among the ≤ 6 months from the 3rd dose group. A recipient should respond to the vaccine within 1-6 month after the 3 dose vaccine series, which is the peak period of the anti-HBs response. As expected, the anti-HBs Ab titers were significantly higher in the ≤ 6 months group than the > 6 months group. Generally, more and stronger associations were observed in the ≤ 6 months subjects than in either the total or > 6 months subjects.
Several studies have shown an association between IL-4 genetic polymorphisms (rs2243250, rs2227284 and rs2070874) and an individual’s response to the HBV vaccine among Asian populations, whereas similar associations were not observed among Caucasian populations.27,28 In contrast to our study, a study of hemodialysis patients revealed a borderline association of the IL-12B rs321227AC gene with positive vaccine responses,29 and a study of adults showed an association of IL-12B rs17860508 with low responses to HBV vaccination.19 Differences among studies investigating the association of cytokine genes with particular disorders are expected and may be due to several reasons, such as differences in study design, sample size, subject ethnicity and source, and genotype methods. IL-4 is an immunomodulatory cytokine secreted by activated Th2 lymphocytes, basophils, and mast cells. This cytokine plays a major role in the modulation of the homeostasis of T lymphocyte subsets. An imbalance between these T lymphocyte subsets has been implicated in the response to therapeutic vaccination in humans.24,30 Thus, genetic mutations in the IL-4 gene may result in abnormal expression and are likely linked to HBV vaccination.31
Cytokine genes are polymorphic and show differences in distribution among ethnic groups. Thus, different linkages of specific cytokine gene alleles with ‘direct causative factors’ may exist. Another probable hypothesis is that the different genotype frequencies among populations obscure or exaggerate the association. Our study has several limitations. Our study covers only a short term period that is not sufficient to observe a long-term immune response to vaccination. Second, we sought to recruit ideal subjects excluding known confounding factors (e.g., asthma, severe infection and atopy) that could alter the immune response profile based on the medical history, but as a retrospective study, the possible occurrence of recall or selection bias might have influenced the reliability of the results. Third, we could not exclude the possibility that other genes located close to the ‘candidate’ genes played a role, and due to the lack of functional studies, we could not conclusively demonstrate the role of the IL-4 gene in the context of HBV vaccine. Although the exact function and effects of IL-4 genetic polymorphisms on the response to the HBV vaccine are not yet clear, we predicted an association with anti-HBs Ab production based on the known functions of IL-4 and several reports of IL-4 association with other vaccination responses.
In conclusion, we presented that the IL-4 rs2243250 and IL-4 rs2227284 polymorphisms have an association with anti-HBs Ab production after vaccination in healthy Korean infants. This study is the first to investigate the underlying mechanisms related to cytokine gene behind poor immune response to HBV vaccination in Korean infants.











 text new page (beta)
text new page (beta)