Introduction
Enterococcus genus are Grampositive cocci, generally grouped in pairs or short chains. They are facultative anaerobes, catalase-negative, and have Lancefield group D oligosaccharides. Their primary habitat is water, soil, and food; above all, they are part of animal (including) intestinal microbiota (Ortega-González, 2010). The Enterococcus genus include species associated with human diseases whose prevalence in food is mainly due to their resistance to adverse environmental conditions (Marguet et al., 2008; Chajęcka-Wierzchowska et al., 2017). Enterococcus faecalis and E. faecium are the two main species involved in human infections such as bacteremia, infective endocarditis, wound infections, and urinary tract infections, among others (Alzahrani et al., 2022; Hammerum 2012; Hayes et al., 2004), and they are resistant to antimicrobials. Enterococcus is inherently resistant to several first-line antimicrobial agents; they show low-level resistance to β-lactams and aminoglycosides (Alzahrani et al., 2022). Therefore, controlling bacterium infections and avoiding cross-contamination with food is of utmost importance (Hammerum, 2012).
Since the detection of vancomycin-resistant Enterococcus and streptogramin-esistant E. faecium outside hospitals, molecular typing methods have been implemented to compare enterococci from different sources. The genetic profiles obtained gave a clearer picture of the isolates involved and a comparison of the clonal transfer of plasmids (Hammerum, 2012).
Molecular techniques are preferable to phenotypic ones due to their better reproducibility, typing power, versatility, and discrimination. They are widely used as a complement to conventional epidemiological investigations, for the study of nosocomial infections of bacterial etiology as well as in the epidemiological study of different bacterial isolates distribution, based on genetic profiles (Corrales and López-Cánovas, 2016). One of the molecular techniques proven useful to precisely distinguish between E. faecalis and E. hirae isolates, and to reveal the genetic diversity of E. faecalis isolates from birds is the amplification of intergenic repetitive consensus sequences (ERIC-PCR). In addition, it has shown great discriminating power between cheese E. faecium isolates, compared to other techniques such as pulsed-field gel electrophoresis (PFGE). Therefore, it is useful to obtain a bacterial genetic distribution and epidemiology (Blanco et al., 2017). Also, in other studies, ERIC-PCR has shown a good differentiation power for E. coli molecular typing (Ardakani and Ranjbar, 2016; Ramakrishnan et al., 2022).
Thus, the aim of this study was to determine the anti-biotic susceptibility and the genetic profiles using ERIC-PCR of Enterococcus spp. previously isolated from commercial chicken viscera in order to observe the relationship of these isolates.
Materials and methods
Strain reactivation
Enterococcus samples isolated from chicken viscera purchased in retail stores from Hermosillo, Sonora, México, were conserved by deep freezing (-80 °C). Vials were defrosted at 25 °C, placed into BHI broth, and incubated at 36 °C for 24 - 48 h for reactivation. Initially, every chicken viscera was evaluated according to the Official Mexican Norm NOM-210-SSA1-2014 (DOF, 2015) at the University of Sonora Microbiology laboratory, for the obtention of Enterococcus. All the positive and confirmed Enterococcus isolates were used in this study.
Phenotypic characterization
Biochemical tests were performed on the previous isolates to observe the typical characteristics of the genus Enterococcus. The tests carried out were Gram staining, catalase, growth in salty BHI broth, and Bile Esculin Medium (BEM) (Devriese et al., 1993). Also, the carbohydrate fermentation test was carried out according to the recommendations of García-Galaz et al. (2004). Sorbose, lactose, adonitol, melezitose, melibiose, raffinose, arabinose, glucose, galactose, sorbitol, sucrose, trehalose, inulin, xylose and mannitol were used.
Antimicrobial resistance determination
The disk diffusion method was used according to the recommendation of the Clinical & Laboratory Standards Institute (CLSI, 2020) to evaluate the isolates resistance to the following antimicrobials: ampicillin (10 µg), streptomycin (10 µg), gentamicin (10 µg), amikacin (30 µg), kanamycin (30 µg), amoxicillin/clavulanic acid (20 +10 µg), erythromycin (15 µg), ciprofloxacin (5 µg), norfloxacin (10 µg), nitrofurantoin (100 µg), imipenem (10 µg), tetracycline (30 µg) and vancomycin (30 µg). Staphylococcus aureus (ATCC 25923), E. faecalis (ATCC 29212) and E. faecalis (ATCC 51299) were used as control strains. The assay was performed in Mueller-Hinton Agar (Difco), and the inoculum of each bacterium was obtained by the absorbance 665 nm (0.08 - 01) using a spectrometer (Thermo Spectronic 40001/4).
Genomic characterization by ERIC-PCR
DNA extraction. Once the isolates were phenotypically characterized, their genomic DNA was extracted according to Wright et al. (2017) with some modifications using lysozyme (24000 kU/mL, Sigma, Canada) and mutanolysin (40 kU/mL). The DNA integrity was checked through a 1.0 % agarose gel electrophoresis and developed with GelRed (Biotum, U.S.A.) under ultraviolet light. Subsequently, the bacterial genetic material was quantified using an AquaMate Vis spectrophotometer (Thermo Scientific).
ERIC-PCR. Amplification of the DNA extracted from each isolate was carried out by the ERIC-PCR technique. The reaction mixture contained 200 µM of dNTP’s, 0.4 µM of ERIC- 1 (5’-ATGTAAGCTCCTGGGGATTCAC-3’), 0.4 µM ERIC-2 (5’-AAGTAAGTGACTGGGGTGAGCG- 3’), 2 mM of MgCl2 (Pro-mega, USA), 5 µL of 5X buffer with dye (Promega, USA), and one U of Taq DNA polymerase (Promega, USA). Then, sterile deionized water was added to complete a final volume of 20 µL and gently homogenized. Finally, 5 µL of previously extracted DNA (containing 100 ng) were added to each microtube with the reaction mixture (Modified from Cabral et al., 2012; Durmaz et al., 2015). After obtaining the ERIC-PCR results, the profiles were visualized or checked through a 1.5 % agarose gel electrophoresis and developed with GelRed (Biotum, U.S.A.) under ultraviolet light, and the image captured by the photodocumentator WiseDOc (WGD-305, Korea). The fragments size was estimated and compared with the 100-bp molecular ladder.
Statistical analysis
The results of the carbohydrate fermentation test and the ERIC-PCR profiles were analyzed with the BioNumerics version 6.5 software (Applied Maths, Belgium), using Pearson or UPGMA (Unweighted Pair Group Method with Arithmetic Mean) to make the clusters and the DICE coefficient (80 %), which provides a percentage of similarity among the Enterococcus isolates. Furthermore, the ERIC-PCR results were correlated with the determination of antibiotic resistance through the disk diffusion test (Durmaz et al., 2015).
Results and discussion
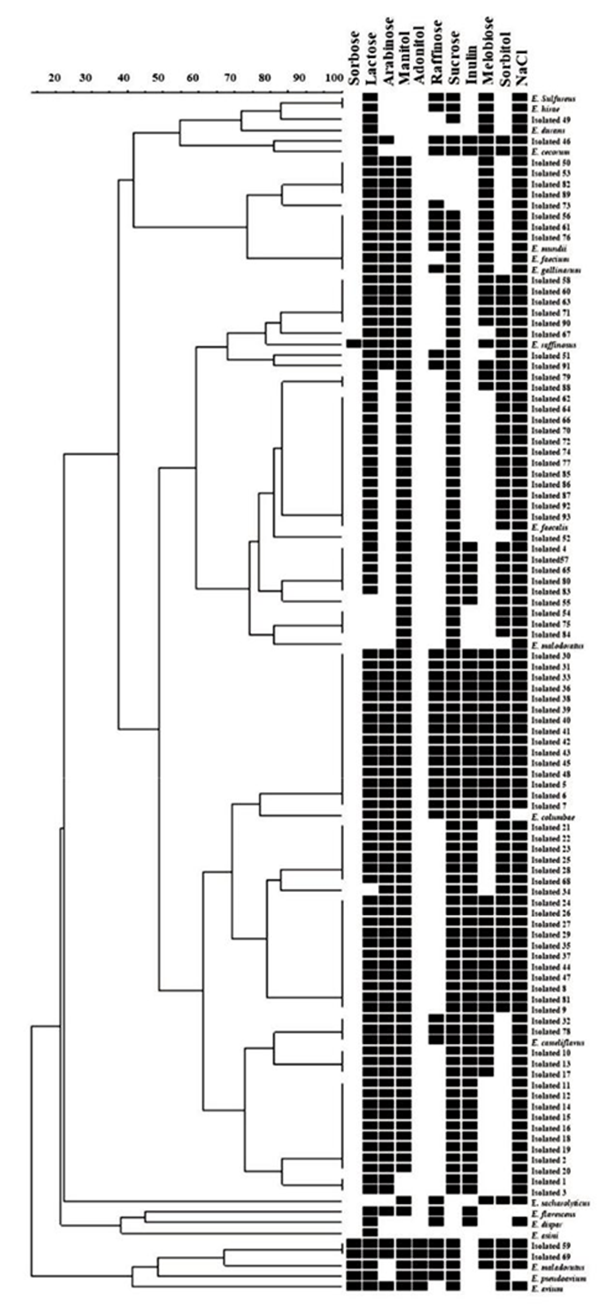
A total of 73 isolates were confirmed to belong to the Enterococcus genus. The carbohydrate fermentation results gave the presumptive phenotypic characterization of the species, and by means of the BioNumerics program output, it was possible to form isolates groups, a dendrogram (Figure 1). It allowed us to relate and discriminate the species with more than 60 % similarity. According to the prevalence (%) for each Enterococcus species, E. casseliflavus and E. faecalis showed the highest prevalence, corresponding to 53 % (39 isolates) and 20.5 % (15 isolates), respectively. The rest of the species showed lower than or equal to 6 %, as shown in Table 1.
Table 1 Enterococcus species isolated from chicken viscera identified by biochemical characteristics.
Tabla 1. Especies de Enterococcus aisladas de vísceras de pollo, de acuerdo con sus características bioquímicas.
| Identified species | Total isolated | % Isolated |
|---|---|---|
| E. casseliflavus | 49 | 53 % |
| E. faecalis | 21 | 23 % |
| E. raffinosus | 8 | 9 % |
| E. faecium | 5 | 5 % |
| E. solitarius | 3 | 3 % |
| E. mundtii | 3 | 3 % |
| E. malodoratus | 2 | 2 % |
| E. cecorum | 1 | 1 % |
| E. durans | 1 | 1 % |
| Total | 73 | 100 % |
In chickens, it has been observed that an agedependent succession of enterococcal species colonizes the intestines. Initially, they are colonized by E. faecalis, then displaced mainly by E. faecium, and afterwards, these species are replaced by E. cecorum in adult chickens (Aarestrup et al., 2002; Lebreton et al., 2014). Nevertheless, Lebreton et al. (2014) indicate that E. faecalis is also found in young chickens. For this reason, the E. faecalis species found in our study could be due to human contamination during handling the product since they were not young chickens.
Our results differ from those reported by Hidano et al. (2015) and Alzahrani et al. (2022), who isolated Enterococcus species from chicken product samples. They found that E. faecalis (75 and 50 %) dominated as the high-prevalence species followed by E. faecium (16 and 33 %). This contrasts with our results since E. faecalis appeared with high frequency, whereas E. faecium had a prevalence near 5 %.
On the other hand, E. cecorum was initially described as part of the normal microbiota in poultry, which coincides with the findings in this work. Even if it was found in low proportion, it is recognized as an important pathogen in feedlots. In the case of humans, it has had sporadic participation in infections such as septicemia, endocarditis and osteomyelitis (Hayes et al., 2004).
Gallegos and Guerrero (2004) performed a similar study in which Enterococcus was isolated from chicken viscera. They found that 23 % of isolates belonged to E. casseliflavus, also reporting E. cecorum (22.8 %), E. gallinarum (19.4 %), E. sulfureus (10.1 %), Enterococcus spp. (8.4 %), E. avium (6.7 %), E. malodoratus (5.9 %), E. faecalis (1.6 %) and E. hirae (0.8 %). These results partially agree with the data obtained in our study. The diversity of species is similar, E. casseliflavus was predominated in both cases, but there is dissimilarity in the other species prevalence, which might be due to the raising poultry practices, that can modify the Enterococci species in the microbiota.
Resistance to Antibiotics by Disk Diffusion
Results of the resistance profiles determined by the disk diffusion test are presented in Table 2. The antimicrobial resistance demonstrated by all the isolates to antibiotics was notorious for amikacin (42 % of the isolates), kanamycin (38 %), streptomycin (55 %) in addition to erythromycin (33 %). These results indicate that the susceptibility to the antimicrobials is low and a large number of the isolated showed intermediate susceptibility. Also, it is noteworthy the representative number of isolated resistance to vancomycin (22 %), which is interesting since these isolates were obtained from chicken viscera, not from a hospital source, where phenotypes with resistance to this antibiotic are commonly found. The results in this work are similar to those reported by Alzahrani et al. (2022), where resistance to tetracycline (55.6 %), erythromycin (31.1 %), ciprofloxacin (21.1 %) ampicillin (30 %) and nitrofurantoin (17.8 %) were the most frequent, except the last two antibiotics which in our work, showed low resistance. In theory, the chicken viscera isolated have not been exposed to antibiotics. However, it is possible that the human handling of the product in the supermarket was the contamination source.
Tabla 2. Susceptibilidad a antibióticos en Enterococcus spp. analizados a través del método de disco difusión.
| Drug group | Antibiotic | % Resistant (n) | % Intermediate (n) | % Susceptible (n) |
|---|---|---|---|---|
| 1. Aminoglycosides | AMK | 42 % (31) | 14 % (10) | 44 % (32) |
| KAN | 38 % (28) | 35 % (26) | 26 % (19) | |
| STR | 55 % (40) | 20 % (15) | 25 % (18) | |
| 2. Aminopenicillins | AMC | 8%(6) | 0%(0) | 92 % (67) |
| AMP | 2%(3) | 1%(1) | 96 % (70) | |
| 3. Carbapenems | IMP | 3%(2) | 0%(0) | 97 % (71) |
| 4. Fluoroquinolones | CIP | 18 % (17) | 27 % (20) | 55 % (40) |
| NOR | 14 % (10) | 9%(7) | 77 % (56) | |
| 5. Glycopeptides | VAN | 22 % (16) | 21 % (15) | 57 % (42) |
| 6. Macrolides | ERI | 33 % (24) | 38 % (38) | 15 % (11) |
| 7. Nitrofurans | NIT | 10 % (14) | 9%(7) | 84 % (61) |
| 8. Tetracyclines | TCY | 22 % (16) | 4%(3) | 74 % (54) |
AMK: Amikacin; KAN: Kanamycin; STR: Streptomycin; AMC: Amoxicillin/clavulanic acid; AMP: Ampicillin; IMP: Imipenem; CIP: Ciprofloxacin; NOR: Norfloxacin; VAN: Vancomycin; ERI: Erythromycin; NIT: Nitrofurantoin; TCY: Tetracycline. The bacterium susceptibility (Resistant, Intermediate and Susceptible) was determined based on CLSI recommendations and the disk fabricant of each antimicrobial, using control strains.
AMK: Amikacina; KAN: Kanamicina; STR: Estreptomicina; AMC: Amoxicilina/ácido clavulánico; AMP: Ampicilina; IMP: Imipenem; CIP: Ciprofloxacino; NOR: Norfloxacino; VAN: Vancomicina; ERI: Eritromicina; NIT: Nitrofurantoina; TCY: Tetraciclina. La susceptibilidad de las bacterias (Resistente, Intermedio y Susceptible) fue determinada basada en las recomendaciones del CLSI y las del fabricante de cada antimicrobiano, utilizando las cepas control.
Moreover, it is acknowledged that different types of antibiotics are used in poultry raising, mainly those that belong to macrolides (erythromycin), trimethoprim, fluoro-quinolones and tetracyclines, either in a therapeutic or sub-therapeutic way. For this reason, the resistance shown may be due to the chicken viscera samples obtained. Poultry may have been exposed to antibiotics belonging to any of these groups. However, it should be noted that enterococci have intrinsic resistance to macrolides (Novais et al., 2013; Daniel et al., 2015; Woolhouse et al., 2015).
The results of the disk diffusion test shows that the isolates are multidrug-resistant. However, one isolate showed resistance to eight antibiotics and was characterized as Enterococcus faecium. Its high percentage of resistance to vancomycin is worrisome because it is a naturally multi-resistant bacterium, in which daptomycin and linezolid resistance also begins to appear. In some cases, this has led to a total absence of effective antibiotics (Alós, 2015).
The multidrug resistance among Enterococcus species may be due to their intrinsic resistance to aminoglycosides and β-lactams, or because they can easily acquire resistance from the environment where extrinsic resistance is found.
Due to the massive use of antibiotics, a highly significant increase in the prevalence of resistance has been observed worldwide (Cercenado, 2011; Alós, 2015).
The appearance of MDR (Multidrug-resistant) bacteria (in this case Enterococcus) in livestock and poultry industry is a public health concern due to the reported transmission of these bacteria to humans. The spread of MDR bacteria occurs by consuming contaminated food, direct contact with farmers and veterinarians or indirectly by handling animal waste, contaminated soil, water, or surfaces. Resistance determinants, carried by MDR strains, can also be transmitted to other commensal isolates in the host and cause future complications. In addition, infections caused by MDR isolates have been associated with extended hospital stays, high morbidity and mortality rates (Daniel et al., 2015; Price et al., 2018; Beganovic et al., 2018).
It is known that the microbiota plays a fundamental role in the regulation of the organism, and that the type of microbiota depends on various factors, including diet. Currently, selective microbiota associated with the consumption of contaminated products with resistant bacteria has been observed, which is also of great concern. Additionally, bacteria such as enterococci can easily acquire and transfer resistance. For this reason, it is essential to know the profiles of resistance and proper of use antibiotics, whether in human, veterinary, or animal husbandry medicine.
ERIC-PCR
All the isolates showed around 2 - 11 bands in the ERIC-PCR test. Most of them resulted in 3 (23.29 %), 4 (28.77 %), or 6 (13.70 %) bands. The ERIC segment size was 100 to 4,361 bp, coinciding with those reported by Wijetunge et al. (2012) who characterized E. cecorum species isolated from diseased poultry, and obtained between 2-7 bands with a size of 200-5000 bp, similar to those obtained in our study.
The band analysis from the ERIC-PCR products electrophoretic gels, through a dendrogram obtained by UPGMA analysis using the DICE coefficient with a similarity value of 80 %, is shown in Figure 2. As can be seen, the analysis shows a high genetic variability among the isolates, which coincides with the findings of Wijetunge et al. (2012) and Blanco et al. (2017).
Most of the clusters had around 20 and 60 % similarity in the dendrogram analysis. Based on this, they were classified into 10 groups (I to X). Group I includes two E. casseliflavus isolates with 100 % similarity and one group of an isolate with ≥ 80 % similarity. Group II contains two isolates with 100 % similarity (one E. casseliflavus, and the other E. solitarius), and two groups with ≥ 80 % similarity. Group III comprises two groups with ≥ 80 % similarity, each with two isolates. Groups IV and V have only one group with ≥ 80 % similarity containing two isolates each. Groups VI to X contain isolates that do not have a significant percentage of similarity, which is why they are considered different. This analysis shows the genomic diversity among the isolated Enterococcus, even among the specie characteristics determined by biochemical tests and carbohydrate fermentation.
Figure 3 shows the dendrogram that includes the analysis of the ERIC-PCR genomic profiles and antibiotic susceptibility tests. In this analysis, the two E. casseliflavus isolates with 100% similarity in group I coincide in presenting resistance to kanamycin, erythromycin and streptomycin. On the other hand, they have ≥ 80 % similarity with another E. casseliflavus isolate, sharing resistance to kanamycin and erythromycin. In group II are two other isolates (E. casseliflavus and E. solitarius) with 100% similarity. This resistance profile coincides with amikacin, kanamycin, erythromycin, vancomycin, and streptomycin. Group III presents two similar isolates (E. casseliflavus and E. raffinosus) with resistance to kanamycin, erythromycin and streptomycin; and two other isolates (E. mundtii and E. faecalis) that have resistance to erythromycin, tetracycline, vancomycin and streptomycin.
Figure 3 Dendrogram obtained from the ERIC-PCR and antimicrobial profiles analysis of 73 Enterococcus species isolated from chicken viscera. AMK: Amikacin; KAN: Kanamycin; STR: Streptomycin; AMC: Amoxicillin/clavulanic acid; AMP: Ampicillin; IMP: Imipenem; CIP: Ciprofloxacin; NOR: Norfloxacin; VAN: Vancomycin; ERI: Erythromycin; NIT: Nitrofurantoin; TCY: Tetracycline.
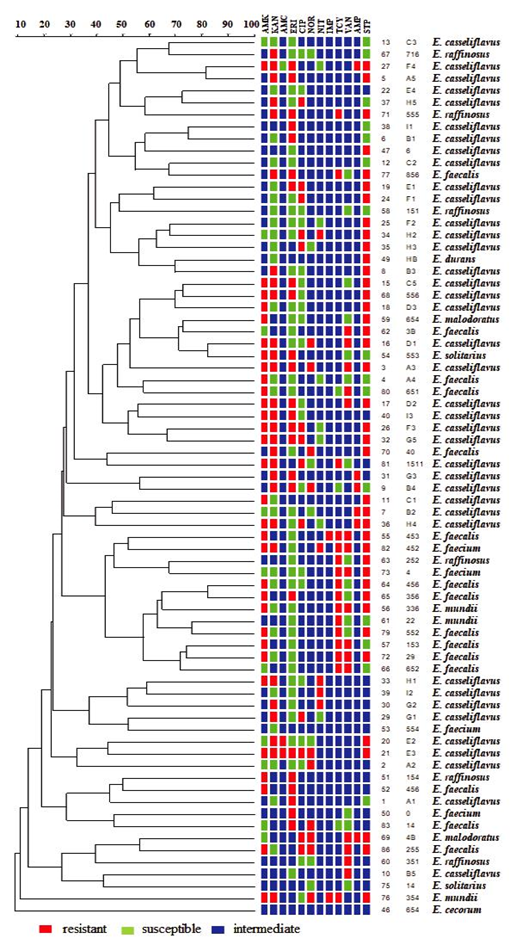
Figura 3. Dendrograma obtenido del análisis por ERIC-PCR y de perfiles de susceptibilidad a antimicrobianos de 73 especies de Enterococcus aisladas de vísceras de pollo. AMK: Amikacina; KAN: Kanamicina; STR: Estreptomicina; AMC: Amoxicilina/ácido clavulánico; AMP: Ampicilina; IMP: Imipenem; CIP: Ciprofloxacino; NOR: Norfloxacino; VAN: Vancomicina; ERI: Eritromicina; NIT: Nitrofurantoina; TCY: Tetraciclina.
In group IV, there are two isolates (E. casseliflavus and E. faecalis) with resistance to amikacin, kanamycin, erythromycin, ciprofloxacin and streptomycin. Group V contains two similar isolates (E. faecalis and E. casseliflavus) with resistance to kanamycin and norfloxacin.
The similarity percentage used for the dendrogram analysis was ≥ 80 %. Fifteen isolates had a resistance percentage higher than or equal to 80 %, showing a great diversity of strains among the isolates, even of the same species according to the phenotypic characterization. As mentioned above, this was perhaps due to the product handling in the different supermarkets where poultry viscera were obtained. Also, the chicken samples could come from different farms and/or companies.
These results agree with those of Bedendo and Pignatari (2000), where identical profiles were observed through molecular techniques in E. malodoratus and E. hirae samples. They stated that this may indicate that conducting studies only at the phenotypic level, such as biochemical tests or susceptibility to antibiotics, does not provide accurate results about the species. Also, that among different species, it is possible to obtain an identical band, thus both, phenotypic and genotypic studies, complement each other. The molecular techniques used to typify bacterial strains are known to be statistically significant in discriminatory power and reproducibility, standardization, and interpretation, making them indispensable in any epidemiological study (Palomino-Camargo and González-Muñoz, 2014).
The isolates related through the ERIC-PCR profile also showed a relationship with their antibiotic resistance profile. This has been previously reported in enterococcal isolates from hospitalized patients, which were related to their ERIC band profile and antibiogram. A similar resistance pattern among isolates classified in the same group could indicate that they came from a common site and have both chromosomal genes and resistance mechanisms (Zalipour et al., 2019).
Conclusions
This study shows the prevalence of E. casseliflavus isolates in chicken viscera. A high percentage of antimicrobial resistance was observed to aminoglycoside and macrolide antibiotics; also, the isolates had a worrying resistance percentage to vancomycin. The ERIC-PCR analysis showed high genetic variability among the isolates and a correlation between the antimicrobial and ERIC-PCR profiles.











 nueva página del texto (beta)
nueva página del texto (beta)