Introduction
The family Magnoliaceae consists of 12 genera with approximately 210 flowering plant species (Morshedlo et al., 2017). Magnolia grandiflora is a genus distributed in tropical-subtropical regions on America and Asia as ornamental trees, with economic importance as a source of aromatic large cup-shaped flowers (Lee et al., 2011; Farag and Al-Mandy, 2013). It has been used in American, Asian and Indian traditional medicine for centuries with attributed properties such as anxiety, nervous disturbance and pain controller, as well as antiseptic agent, diaphoretic, anti-inflammatory, antiseptic, and stimulant agent (Latif et al., 2017; Ma et al., 2020). In Mexican traditional medicine it has been used as treatment against epilepsy, spams, inflammatory and infertility diseases (Dominguez-Yescas and Vázquez-García, 2019).
Many reports state that different plant structures of M. grandiflora contain active compounds such as sesquiterpenoides (Hong et al., 2007), coumarins (Hussein and El-Anssary, 2019), phenylpropanoids (Cao et al., 2021), lignans (Schühly et al., 2009), glycosides (Wang et al., 2019), alkaloids (Cho et al., 2022), among others (Lim, 2014). These compounds have demonstrated biological activities such as antitumor (Chilampalli et al., 2011), antimicrobial (Chang et al., 1998), anti-inflammatory (Kim and Cho, 2008), anti-tyrosinase (Huang et al., 2012), anti-allergic (Niitsuma et al., 2001), cardioprotective (Ho and Hong, 2012) and antiviral (Lan et al., 2012) activities. Moreover, in the cosmetic industry, M. grandiflora is used because of its anti-inflammatory and anti-acne activities exerted by its biphenols magnolol and honokiol active compounds (Mukherjee et al., 2011).
Flowers of M. grandiflora are an important source of essential oils which are extracted with aqueous and alcoholic based techniques (Davé et al., 2012). These volatile floral substances are mainly monoterpenoids, sesquiterpenoids and phenylpropanoids formed by the mevalonato-methyl erythritol phosphate (MEP) and shikimate pathways (Averesch and Krömer, 2018). The most common reported chemical constituents in M. grandiflora flowers are β -elemene, ger-macrene, bicyclogermacrene, D, β -elemene, (E)- β-ocimene, β-Caryophyllene, cyclocolorenone and geraniol (Baez et al., 2012; Davé et al., 2012; Lim, 2014; Morshedloo et al., 2017). The antioxidant capacity exerted by many of these compounds was effective against cancerous cell proliferation without cytoxiticy (Li et al., 2009; Farag and Al-Mahdy, 2013; Raut and Karuppayil, 2014).
Nonetheless, phytochemical studies reported that the extracted essential oils are chemically different and remarkably variable in their qualitative and quantitative compositions (Morshedloo et al., 2017) due to methods of extraction, environmental conditions, developmental stages of flowers and genetic factors (Lim, 2014). Moreover, further studies regarding chemical compositions, antioxidant capacity and cytotoxicity of Mexican M. grandiflora flowers to evaluate its potential applications are necessary (Sánchez-Recillas et al., 2014; Vázquez-García et al., 2015). Hence, the objective of the present study was to characterize a) the chemical composition, b) the antioxidant activity and c) cytotoxicity effect of two M. grandiflora flower extracts.
Materials and methods
Plant material and chemicals
Flowers of M. grandiflora were collected during a rain period on June 2018 from cultivated trees in the Orizaba, Veracruz Faculty of Chemical Sciences at Universidad Veracruzana. The collected flowers were located on tomentose pedicels, erect, solitary, large, up to 20 cm in diameter. They had 6-12 white petals, narrowed at the base, and three sepals with a petaloid appearance, as well as both reproductive organs in the same flower. Guidelines on Good Agricultural and Harvesting Practices (GAP) for medicinal plants were followed (WHO, 2003). All the chemicals and solvents were obtained from Sigma-Aldrich (St. Louis, MO).
Plant extracts preparations
The M. grandiflora flowers were washed and then air-dried. The plant material was homogenized in a mortar and then sieved using a 3.2 mm sieve. In order to obtain 2 % (m/v) aqueous and ethyl extracts, 2 g of plant material were mixed with 100 mL of double distilled water (DDW) or 100 mL of ethyl alcohol (96 %), respectively. The aqueous extract was heated to boil for 10 min, filtered with qualitative filter paper disks (Munktell, grade 388), and then concentrated by lyiophilization for a week (lyophilizer, LABCONCO). The ethyl extract was hot continuous percolated over 72 h using Soxhlet apparatus, filtered with qualitative filter paper disks, and then concentrated under reduced pressure in a rotary evaporator (Buchi, RE111). The flower plant extracts were stored in amber flasks at 4 °C until use.
Preliminary phytochemistry of plant extracts
The preliminary phytochemical properties of the flower extracts were examined for the presence of alkaloids (Dragandroff´s reagent, Wagner´s reagent and Sonneschain´s reagent) flavonoids (Shinoda test and Zinc-Hydrochloride test), glycosides (Legal test, H2SO4 test and Borntrager`s test), saponins (Froth forming test), tannins (FeCl3 test, Vanilin-Hydrocloride test and alkaline test) and triterpenoids (Liberman test, Salkowsky test and Noller test), as described by Okeulu and Chinwe (2001). All tests were performed in triplicate.
Qualitative analysis by thin layer chromatography
Thin layer chromatography (TLC) on analytical plates over 10 cm x 20 cm silica gel 60 (Art. 1.05641) was performed in order to separate the plant extracts metabolites. Different solvent systems with different polarities were prepared and TLC studies performed to select the solvent system for a better resolution. The methodology was performed as follows: 1) Flower extracts solutions were applied on 6 mm bands, 5 mm from the bottom, 12 mm from the left edge, 4 mm apart by means of Linomat IV (Camag) on pre-coated (0.25 mm layer, Merck) TLC plates by using capillary tubes, 2) TLC was developed in a crystal chamber using hexane-Ethyl acetate (5:5 and 2:8), 3) TLC plates were dried with Camag TLC plate heater at 110 °C for 20 min, and observed under ultra violet light at 254 and 365 nm respectively, 4) Then, they were sprayed with iodine and vanillin solutions as spraying reagents, 5) Finally, the development of color in separated bands was analyzed and expressed by its retention factor (Rƒ) with the formula:
UV-VIS and FTIR spectroscopic analysis
Flower extracts were examined under visible and UV light for proximate analysis. For UV-VIS and FTIR spectrophotometer analysis (Karpagasundari and Kulothungan, 2014), the flower plant was centrifuged at 3000 rpm for 10 min and filtered through Whatman No. 1 filter paper with a high-pressure vacuum pump. The sample was diluted 1:4 with the aqueous and methanol solvents respectively. The flower extracts were scanned within a wavelength range of 200-800 nm using a CARY 50 VARIAN (Amsterdam, The Netherlands) and the characteristic peaks were detected. The peak values from the UV-VIS and FTIR were recorded and the analysis repeated twice for the spectrum confirmation.
In vitro antioxidant activity
The flower extracts of M. grandiflora were tested for in vitro antioxidant activity by the standard methods. The total phenols content was quantified by the Folin-Ciocalteu method (Singleton and Rossi, 1965). An aliquot (0.5 mL) of each diluted polar extract was mixed with deionized water (35 mL) and 2.5 mL of Folin-Ciocalteu reagent; after 3 min of incubation, a sodium carbonate solution (20 % in water) (5 mL) was added. The solution was incubated at 70 °C for 20 min and then adjusted to 50 mL with deionized water. The UV-VIS absorbance was read at 750 nm (CARY 50 VARIAN; Amsterdam, The Netherlands). The results were expressed as mg gallic acid equivalent per gram of dry weight (mg GAE/g DW). For calibration curve, gallic acid concentrations were used between 0.05 - 0.5 mg/mL (R2 = 0.99).
The method described by Medda et al. (2021) with some modifications was used to quantify the ABTS radical scavenging activity. The ABTS+ cation radical was produced by mixing ABTS stock solution (7 mM in water) and 2.45 mm potassium persulfate at a 1:0.5 ratio respectively, and stored in the dark at room temperature for at least 16 h before use. The ABTS radical was diluted with water until an absorbance at 734 nm reached a 0.7 value. The ABTS solution was prepared fresh before each analysis. Each flower extract (7.5 µL) was mixed with 1000 µL of ABTS diluted. The reaction mix was incubated for 30 min in the dark at room temperature and the absorbance was immediately read at 734 nm using a CARY 50 Scan UV143 Vis VARIAN spectrophotometer (Amsterdam, The Netherlands). The calibration curve was prepared using a range of 0 - 4 mM (R2 = 0.98) of Trolox reagent. The radical scavenging activity was estimated by the decrease of absorbance and expressed as the Trolox equivalent (TEAC) ABTS per mL of essential oil or component as follows:
Where Cabs is the control absorbance at t = 0 (containing all reagents except the test compound), and Sabs is the sample absorbance of the test compound after 30 min. The results were expressed in µmol TE/g extract. All determinations were performed in triplicate. Antioxidant activity was expressed as IC50, defined as the concentration of the test material required to cause a 50 % decrease in initial ABTS concentration.
The antioxidant activity of the flower extracts was also measured by the stable radical of 2,2-diphenyl-1-picryl-hydrazyl (DPPH) method (Brand-Williams et al., 1995). DPPH radical solution (100 µM) in 80 % (v/v) aqueous methanol was prepared. Test samples were prepared by mixing each flower extract (10 µL) and DPPH solution (190 µL), mixed, and then incubating in the dark at 37 °C for 20 min. All tests were performed in triplicate, with vitamin E as a positive control. The absorbance values were measured at 517 nm against a methanol blank (CARY 50 Scan Uv143 Vis VARIAN; Amsterdam, The Netherlands). Trolox was used as a standard for calibration curve (range between 6 - 21 µM; R2 = 0.97). The % of inhibition was calculated with the formula:
Where Cabs is the control absorbance at t = 0 (containing all reagents except the test compound), and Sabs is the sample absorbance of the test compound after 20 min. The results were expressed in µmol Trolox equivalent for g extract (µmol TE/g extract). Antioxidant activity was expressed as IC50, defined as the concentration of the test material required to cause a 50 % decrease in initial DPPH concentration.
Cytotoxicity bioassay
For determining the cytotoxicity of flower extracts, a brine shrimp (Artemia salina) lethality bioassay was carried out (Krishnaraju et al., 2005). Brine shrimp were hatched using brine shrimp eggs in a conical flask (1 L) with sterile seawater (38 g/L, pH 8.5 adjusted with Na2CO3) with aeration. After 24 h, 15 mL of yeast solution 0.06 % was added to the conical flask as larvae feeding; 48 h after the egg’s incubation, active nauplii free from eggshells were collected, counted and placed in each vial containing 4.5 mL of brine solution. After 24 h of exposure to different concentrations (1 - 5,000 µg/mL) of the flower plant extracts (in triplicate per dose), surviving larvae were counted. The lethality (%) was determined by comparing the mean surviving nauplii and control tubes. The LC50 values were obtained from the best-fit line plotted concentration vs percentage lethality. Potassium dichromate (K2Cr2O7) was used as positive control in the bioassay. Sterile seawater was used as negative control.
Data treatment and statistical analysis
Data are expressed as the means of three biological replicates. Results of phenolic compounds, ABTS+ and cytotoxicity determinations are expressed as the means and standard deviations of three replicates. When needed, results were compared using an analysis of variance (ANOVA) with STATISTICA software (StatSoft 10.0; Tulsa, Ok, USA). Significant difference Fisher test (p ≤ 0.05) was used to compare means.
Results and discussion
Preliminary phytochemistry of flower extracts
In the preliminary phytochemical analysis of the flower extracts, the presence of alkaloids, flavonoids, tannins, glycosides, coumarins, quinones, sesquiterpene lactones, saponins, and triterpenoids were examined (Table 1). The results of the preliminary phytochemical tests were compared in both extracts, differing only in two metabolites. Flavonoids and triterpenoids test showed marked presence on the ethyl extract. Positive results were obtained in the alkaloid reaction using Wagner’s reagent for both samples. Serna-González and Guzman-Vazquez (2010) stated that alkaloids from Magnoliaceae family have relaxing properties. Meanwhile, the reaction for flavonoids and tannins indicates the presence of phenolic compounds which confirms a great variety of biological properties, such as antitoxic, antitumor, antiviral, antimicrobial, anti-inflammatory, antibacterial, antiallergic, fungicidal and insecticidal, among others (Tungmunnithum et al., 2018).
Tabla 1. Ensayos fitoquímicos para determinar metabolitos a partir extractos acuosos y etílicos.
| Metabolites | Test | Water extract | Ethyl extract |
| Alkaloids | Dragandroff | - * | - |
| Wagner | + | + | |
| Sonneschain | - | - | |
| Flavonoids | Shinoda | - | - |
| Zinc-Hydrochloride | + | ++ | |
| Tannins | FeCl3 | ++ | +++ |
| Vanilin-Hydrocloride | - | - | |
| Alkaline | - | - | |
| Glycosides | Borntrager | - | - |
| Legal | - | - | |
| Baljet | - | - | |
| Erlich | - | - | |
| Coumarins | NH4OH | - | - |
| NaOH | - | - | |
| Quinones | NaOH | - | - |
| H2SO4 | - | - | |
| Sesquiterpene lactones | Lactones | - | - |
| Saponins Triterpenoids | Saponins | + | + |
| Liberman | - | - | |
| Salkowsky | - | +++ |
*The preliminary results of secondary metabolites recognition are expressed with the symbols (+) and (-), where (+ + +) indicates a fairly marked presence of the reaction and (-) indicates the absence of the metabolite.
Positive results were obtained for saponins, presenting a moderate content of this metabolite, which has not been reported in studies previously carried out with M. grandiflora. As it is known, in the literature saponins may be associated with numerous biological activities that include anti-inflammatory, antibacterial, antifungal, and antiviral (Troisi et al., 2014). Although the presence in M. grandiflora is moderate, it could have an influence on its anti-inflammatory and antibacterial effects.
Triterpenoids test with the Salkowski reagent fairly marked positive (+++) with the ethyl extract. Triterpenes are from the family of terpenes, and there are previously reported sesquiterpenoids and triterpenes in M. grandiflora (Del Valle et al., 2004). This metabolite was only found in the alcoholic extract, which may indicate that has a higher affinity for alcohol. The previous situation also occurred when performing the glycosides test, giving only positive for the aqueous extract with the Baljet reaction, but negative results were obtained with the alcoholic extract. Glycosides have been used to treat congestive heart failure (Ávalos-García and Pérez-Urria, 2009). This result is related with the study carried out by Del Valle et al. (2004) entitled “Studies of M. grandiflora extracts on guinea pig heart muscle”, which mentions that the crude extracts of leaves and petals of M. grandiflora have a positive inotropic effect for the heart, as well as a coronary vasodilation. According to this, in several regions of Mexico, M. grandiflora is known for its effect on diseases of the heart, which is one of his greatest recognitions in herbal medicine.
Qualitative analysis by thin layer chromatography
Thin-layer chromatography was performed in order to obtain a separation of metabolites present in the flower extracts of M. grandiflora. Hexane: Ethyl acetate was used as the mobile phase, at different concentrations (5:5 and 2:8). Each separate analyte was marked and the distance traveled by each one was measured to calculate its Rf. The values obtained from both extracts are shown in Table 2. The preliminary results of the RF at the 5:5 dilution indicate the possible presence of one isolated analyte, and at the 2:8 dilution the presence of two isolated analytes in both extracts is shown, corroborated with the three repetitions performed, since there is not much difference between their Rf. These 3 different metabolites are due to the fact that the dilutions are of different polarity. The 2:8 dilutions are more polar than the 5:5 dilutions. Thus, we can observe the separation of more analytes. The more retained analytes near the origin tend to be of higher polarity since they are fixedly adsorbed to the active centers of the stationary phase, in this case silica gel, whereas the nonpolar ones will elute more easily (Sherma and Fried, 2003). The advantage of thin-layer chromatography as a preliminary analysis technique for extracts should be highlighted, since it gives us an idea of their composition (Qu et al., 2018).
Table 2 Qualitative analysis by thin layer chromatography results of the Rf obtained both solutions.
Tabla 2. Análisis cualitativo por cromatografía en capa fina de los resultados de la Rf obtenida de ambas soluciones.
| 2:8 Dilution | |||
|---|---|---|---|
| Distance (cm) | Rf* | ||
| Ethyl extract | A1 | 1.5 | 0.254 |
| A2 | 1.7 | 0.309 | |
| A3 | 1.7 | 0.309 | |
| B1 | 4 | 0.678 | |
| B2 | 4.7 | 0.855 | |
| B3 | 4.8 | 0.873 | |
| Water extract | A1 | 1.4 | 0.237 |
| A2 | 1.7 | 0.309 | |
| A3 | 1.9 | 0.345 | |
| B1 | 4.1 | 0.695 | |
| B2 | 4.8 | 0.873 | |
| B3 | 4.8 | 0.873 | |
| 5:5 Dilution | |||
| Distance (cm) | Rf | ||
| Ethyl extract | A1 | 2.4 | 0.421 |
| A2 | 4.1 | 0.745 | |
| A3 | 4.1 | 0.745 | |
| Water extract | A1 | 3.2 | 0.582 |
| A2 | 3.9 | 0.709 | |
| A3 | 3.9 | 0.709 |
The retention factor (RF) is the result of (a) the distance of flower extract divided by (b) the distance of the eluent over the thin layer chromatography. El factor de retención (RF) es el resultado de (a) la distancia del extracto floral dividida por (b) la distancia del eluyente sobre la cromatografía en capa fina.
UV-VIS and FTIR spectroscopic analysis
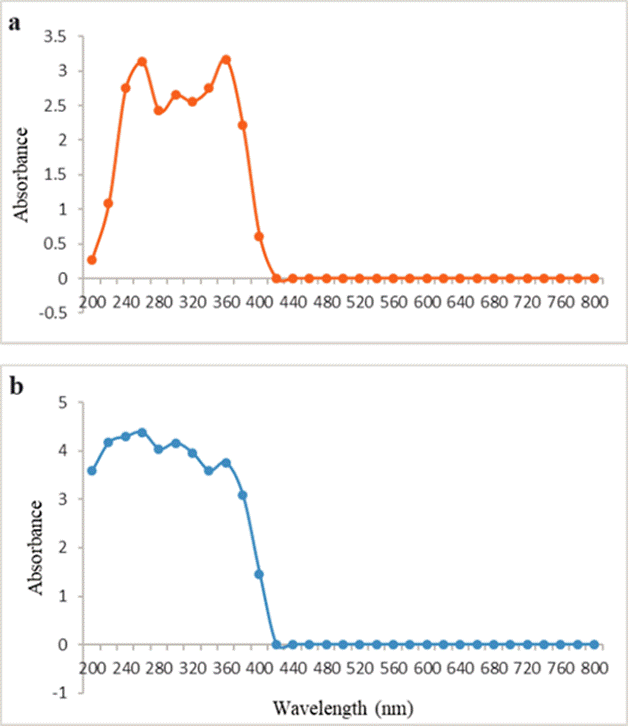
The UV-VIS analysis was performed to identify phytoconstituents present in ethyl and water extracts of M. grandiflora. The mentioned analysis was performed to identify the chemical compounds that contains σ-bonds, π-bonds and lone pair of electrons, chromophores and aromatic rings. The qualitative UV-VIS profile of both M. grandiflora extracts were determined between the wavelengths of 200 nm and 800 nm, in order to obtain a proper baseline and due to the peaks and sharpness. The performed analysis of ethyl extract showed peaks at 260 nm and 360 nm respectively (Figure 1a).
While the performed analysis of water extract showed peaks at 240 nm, 260 nm and 360 nm respectively (Figure 1b). The absorption spectrum of the M. grandiflora flower extract from the 400 nm to 800 nm region was not detectable.
In this analysis the peaks of absorbance at 200 to 400 nm are correlated to the presence of unsaturated groups and heteroatoms such as O, S, N (Njoku et al., 2013). In both extracts, the spectrum shows many peaks from 300 nm to 400 nm which confirms the presence of organic chromophores within the M. grandiflora flower extracts. Nonetheless, the UV-VIS analysis has some limitations by the inherent difficulties to record the peaks to any particular constituents in the system. The UV-VIS results should be complemented with any other analytical technique such as FTIR and GC/MS techniques to a proper extract characterization and constituent identification (Karpagasundari and Kulothungan, 2014).
The FTIR spectrum was performed to identify the functional group of the active components based on the peak absorbance detected at the infrared radiation. The FTIR spectrum of the M. grandiflora flower extracts in the form of wave numbers (cm-1) are shown in Table 3. The wave absorption numbers at 3,348.22 and 3,428.32 are due to the stretching hydroxyl groups (Pramila et al., 2012). The band at 2,930.02 is due to symmetric stretching of saturated (sp3) carbon (Karpagasundari and Kulothungan, 2014). The bands at 1,579.98 and 1,629.78 are assigned to the bending mode of absorbed water, since plant extracts even ethyl extracts are known to have a strong affinity for water (Oliveira et al., 2016). The bands at 1,550.02 and 1,540.02 are due to C=C stretching related to the aromatic structure of both extracts (Al-Shareefi et al., 2019). The vibrational absorption band at 1,402.22 was related to rocking of methyl group (Ashokkumar et al., 2014). A band at 1,253.97 was related to C-O stretching (Oliveira et al., 2016). The bands at 577.97 and 587.97 were due to the aromatic ring out of plane bending (Carballo et al., 2008).
Tabla 3. Características de la estructura de extractos de flores de M. grandi-flora por FTIR.
| Ethyl extract | Wave numbers (cm-1) | Assignments |
|---|---|---|
| 3,348.22 | -OH stretch | |
| 1,579.98 | Alkene C=C | |
| 1,550.02 | C=C stretching | |
| 577.97 | Aromatic ring | |
| Water extract | ||
| 3,428.32 | -OH stretch | |
| 2,930.02 | C-H stretch | |
| 1,629.78 | Alkene C=C | |
| 1,540.02 | C=C stretching | |
| 1,402.22 | C-H bending | |
| 1,253.97 | C-O stretch | |
| 587.97 | Aromatic ring |
In vitro antioxidant activity
Extracts of aromatic plants are known to have anti-oxidant properties, thus this offers the possibility of being used as natural preservatives for food and cosmetics (Bendif et al., 2017). In the present study, the antioxidant activity of M. grandiflora flower extracts was investigated (Table 4). In general, the antioxidant activity of plant extracts is primarily due to phenolic compounds and, in M. grandiflora flower extracts, the presence of different groups of phenolic compounds such as phenolic acids, flavonoids, and diterpenoids, are responsible for the observed antioxidant properties (Elansary et al., 2019). The total phenolic content (TPC) (expressed as gallic acid equivalents) is often used as an approximately measurement of the antioxidant power of a plant extract (Wu et al., 2018). The TPC values reported for both M. grandiflora flower extracts, which were determined by the Folin-Ciocalteu assay, are also reported in Table 4. From the obtained data, slight differences in TPC values can be observed between water and ethyl extracts, with the former exhibiting the highest TPC. These results are consistent with the concentrations of the major chemical compounds showed in Table 1.
Tabla 4. Actividad antioxidante de extractos etílicos y acuosos de flores de M. grandiflora.
| Extracts | Polyphenols (mg GAE/g dw)- | ABTS | DPPH | ||
|---|---|---|---|---|---|
| TEAC* (µmol TE/g) | IC50#(µg/mL) | TEAC*(µmol TE/g) | IC50# (µg/mL) | ||
| Ethyl | 46.8 ± 3.2a‡ | 459.6 ± 8.5a | 39.4 ± 1.5b | 3,210.4 ± 2.5a | 54.5 ± 1.1b |
| Water | 34.3 ± 1.3b | 274.2 ± 5.7b | 51.8 ± 0.9a | 219.7 ± 0.9b | 63.3 ± 0.4a |
| Control | 3.8 ± 0.4c | 3.02 ± 0.2c | |||
*TEAC: Trolox equivalent (TE) antioxidant concentration.
# IC50: The concentration giving a reduction of 50 %.
‡ Each value is the mean of three replicates ± Standard deviation. Different letters mean significant difference (Fisher, p ≤ 0.05).
*TEAC: Concentración antioxidante de Equivalentes Trolox (TE).
# IC50: La concentración necesaria para reducir el 50 %.
‡ Cada valor es la media de tres repeticiones ± Desviación estándar. Letras diferentes significan diferencia significativa (Fisher, p ≤0 .05).
The flower extracts showed moderate antioxidant activity as reported in all the assays where Trolox was used as reference. The greater antioxidant activity observed in ethyl M. grandiflora flower extracts could be attributed to their phytochemical properties (Tables 1-3) and/or to their synergistic effects (Garza et al., 2019). Elansary et al. (2019), also concluded that Magnolia acuminata had the highest antioxidant activity in comparison with other plant extracts; which was attributed to phytochemicals such as catechin and catechin derivatives.
The antioxidant activity of the flower extracts was measured by the ABTS and DPPH methods. The relatively stable nitrogen-centered free radical DPPH is ubiquitously used to measure the scavenging ability of different phyto-chemicals, extracts or essential oils and their antioxidant effects on DPPH radical depend on their hydrogen donating ability (Dastmalchi et al., 2007). The water extract of M. gran-diflora flowers reduced the concentration of DPPH by 72.09 ± 6 % with and efficacy higher than that of the ethanolic extract (63.5 ± 7 %). Vitamin E, included as positive control in the assay, showed the greatest ability to scavenge the DPPH free radical (88 ± 8 %) at the same test concentrations of 5 mg/mL. As previously mentioned, both flower M. gran-diflora extracts have mainly components such as terpenes and phenolic compounds, and it can be assumed that these are responsible for the high percentage of ABTS and DPPH inhibition (Yoon, 2014). Moreover, flower extracts from other Magnoliaceae species have chemical antioxidant compound such as honokiol and magnolol (Yang et al., 2018).
Cytotoxicity bioassay
A brine shrimp (Artemia salina) lethality bioassay was carried out to determine the cytotoxicity of flower extracts. Table 5 shows the LC50 of the flower M. grandiflora extracts. Both, water and ethyl extracts, did not show toxicity with LC50 higher than 1,000 µg/mL, unlike control, as expected, considered as cytotoxic due to a LC50 lower than 100 µg/mL (Yadav and Mohite, 2020). There is information about cytotoxicity test of M. grandiflora flower extracts carried out by Artemia salina bioassays. Nonetheless, Martínez-Báez et al. (2016) found a moderate cytotoxicity with a LC50 of 400 µg/ mL, in a methanolic M. grandiflora plant extract. In our results, the non- cytotoxicity of both flower extracts could be related to some phenolic compounds such as magnolol which has cytoprotective activity (Zhang et al., 2019).
Tabla 5. Prueba de citotoxicidad por bioensayo de letalidad en camarones en salmuera (Artemia salina).
| Flower plant extract | LC50 (µg/mL) |
|---|---|
| Water extract | 1,116 ± 15b* |
| Ethyl extract | 1,285 ± 14a |
| Control (+) | 10.3 ± 1.7c |
| Negative (-) | 0 |
LC50: Lethal concentration to kill 50 % of brine shrimp. LC50 < 100 µg/mL means toxicity level. *Each value is the mean of three replicates ± Standard deviation. Different letters mean significant difference (Fisher, p ≤ 0.05).
DL50: Concentración letal para matar el 50 % de las artemias. DL50 < 100 µg mL-1 significa un nivel de toxicidad. *Cada valor es la media de tres repeticiones ± Desviación estándar. Letras diferentes significan diferencia significativa (Fisher, p ≤ 0.05).\s\s
Conclusions
The preliminary phytochemical test demonstrated the presence mainly of flavonoids, terpenes, tannins and alkaloids in both extracts. The thin layer chromatography, the UV-Vis and FTIR spectroscopic analyses showed the presence of organic chromophores such as flavonoids. The water and ethyl flower extracts showed antioxidant and non-cytotoxic activity. The water and ethyl extracts of M. grandiflora flowers found in southeast Mexico are a promissory source of chemical compounds with the attributed biological activities by the presented results. Nonetheless, ethyl extracts exerted more antioxidant activity. M. grandiflora flower extracts could be used in medicinal and cosmetic industries by their exerted chemical antioxidant properties and by their non-cytotoxic effects.











 nueva página del texto (beta)
nueva página del texto (beta)