Introduction
Synthetic plastics are commonly extracted from petroleum. They are traditionally used as containers due to their high obtainability, low cost and good mechanical properties. However, it is well known that these synthetic materials affect significantly the environment due to their low degradability (Li et al., 2016). Biofriendly materials based on natural sources are the focal point of interest of many researchers, due to their advantages such as ecological nature and its reusability (Mittal et al., 2016). The elaboration of films made from polysaccharides and proteins can play an important role in the future of the preservation and packaging of foods (Aila-Suárez et al., 2013). Chayotextle (Sechium edule) is a native cucurbit plant of Mexico. Its roots commonly called chayotextle are edible and contain plenty of starch (89.1%) with the amylose/amylopectin ratio of 12.09 % and 87.10 % respectively. It possesses an alkaline pH value of about 8.12 and lower gelatinization temperature (Jiménez-Hernández et al., 2007). Few studies have been conducted on the isolation (Hernandez-Uribe et al., 2011) and characterization (Aila-Suárez et al., 2013) of this new biopolymer. On the other side, gelatin is different from other hydrocolloids because of its properties as a digestible protein (Duconseille et al., 2015) and film formation capacity (Hosseini et al., 2016). Gelatin is extracted form a variety of sources, such as animal skin, bones, and connective tissues. It is produced by the partial hydrolysis of native collagen containing between 85 and 92 % protein content.
The mixture of starch with gelatin, has been reported to increase the mechanical properties of films in terms of higher resistance and elongation (Moreno et al., 2017). Starch is a polysaccharide widely used worldwide, despite this advantage, few studies have been conducted on chayotextle starch which is considered as a new and innovator biopolymer with different applications. Films prepared with starch possess some disadvantages such as fragility, hydrophilicity, poor resistance to external factors and mechanical properties. However, starch films have advantages as a potential packaging material: natural, renewable, widely available, non-toxic, biodegradable and cheap (Zarski, 2021). Also, the films prepared with gelatin are an ideal material for food packaging due to its versatile advantages such as biodegradability, as well as antibacterial and antioxidant properties in foods. Nevertheless, gelatin films possess poor waterproof and mechanical properties (Lu, 2022). The main purpose of this study consisted in the development of a biomaterial prepared with a blend of Chayotextle starch and gelatin. Also, some properties such as mechanical, molecular, permeability and solubility were investigated.
Materials and methods
For the preparation of films, the followings materials were used: Wilson PS 30 mesh gelatin with 275°B (Gelita, Mx), chayotextle starch and glycerol (Meyer, Mx).
Preparation of films
Isolation of chayotextle starch powder was carried out according to the methodology developed by Hernandez-Uribe et al. (2011). Five treatments of filmogenic solution were prepared according to Table 1.
Firstly, 160 mL of distilled water were added to a mini-reactor (300 mL) followed by 2 g of glycerol and 10 min of stirring (Lightning Lab MasterSPX-Mixer). Different treatments of 4, 3, 2, 1, and 0 g of chayotextle starch were added to the corresponding reactor. The temperature solution was raised at 85 ºC for 10 min. The solution was cooled down to 60 ºC to add gelatin. Secondly, 0, 1, 2, 3 and 4 g of porcine gelatin with 275°B (Wilson Porcine Skin) were placed in a corresponding flask containing 20 mL of water at room temperature for swelling of gelatin. After 10 min of swelling, the temperature of the gelatin solution was increased to 55 ºC and maintained for 10 min with constant stirring for melting of gelatin. The melted gelatin was added to the starch-glycerol solution and stirred for 10 min to homogenize the mixture.
From these gelatin-starch-glycerol mixtures, 60 g of each filmogenic solution were placed in 13 cm-diameter petri dishes. Once the solutions cooled down at room temperature, they were dried in a digitally controlled drying oven (Felisa) at 25 ºC for 72 hrs. Then, films were peeled off from the substrate and cut into strips (10 mm x 100 mm). Average thickness was 0.1 ± 0.005 mm.
Conditioning of samples
Films were stored for seven days over P2O5 (0 % R.H.) for a maximum removal of water in a plastic desiccator with a button lid (Thermo Scientific®). After this storage time, films reported 2.3 ± 0.1 % dry basis of water content. Then, strips were stored over a salt solution (K 2CO3= 44 % R.H.) in a sealed box for 7 days to equilibrate the water content at 15 % dry basis. After the conditioning time, samples were ready for mechanical and structural properties testing.
Tensile strength performance test
The films were clamped in the texture analyzer machine (Brookfield CT3 Analyzer). The distance between tensile grips was 50 mm. It was considered as the original length of the sample for strain calculation. Settings for the machine were; test mode = tension; test speed = 0.10 mm/ sec; distance = 30 mm. The performance of the mechanical properties of films was assessed by stretching the sample from its original length, until the sample was fractured.
Calculations
The resistance of the films to elongation at small deformation is called Young’s Modulus (E) (Sperling, 2006). This was calculated from the linear region of the curve at 0.5 mm of strain as follows in equation 1:
Where: E = Young’s Modulus (GPa); Gradient = Slope of the curve at 0.5 mm of strain (N/mm); ∆L = increment of length (mm); t = thickness (m); w = width of the film (m).
The stress at break and strain at break were obtained from the maximum force and distance of the tensile strength curve.
Fourier Transform Infrared
The absorption spectres were obtained on a Frontier FT-IR (Perkin Elmer) equipped with attenuated total reflectance (ATR). The interval of measurement was from 400 to 4,000 cm-1 at room temperature. The automatic signals were recollected in 3260 scans at a resolution of 1 cm-1 and digitalized with the spectrum 10 software.
X-Ray
The grade of crystallinity was measured in a Bruker (D2 PHASER Second generation) diffractometer. Films samples were cut into 2.4 cm diameter-circles. A divergence apparatus was used at 0.6 mm and the data recollected were in a range of 2ϴ from 5 ° to 80 ° at a speed of 1°/ min; 30 mA and 40 kV. The calculations of both amorphous and crystalline content of films were calculated with the Diffrac.suite-EVA software.
Solubility and permeability of films
Previous to the solubility test, samples were stored in a desiccator for 7 days to remove water. The solubility was performed according to Garcia et al. (2004), with some modifications. Samples sized 2 x 3 cm were weighed and placed in glass vessels containing 80 mL of distilled water and kept under constant stirring for 1 h at 25° C. Samples were dried at 70 °C for 2 h. The solubility percentage was calculated with the following equation:
The permeability to water vapor in films was determined employing the standard gravimetric method of the ASTM, E 96-80 (ASTM, 1989) better known as “the test cell”.
Statistical analysis
A randomized design experiment and an analysis of variance (ANOVA) were applied to experimental data which included a Tukey test (P ≤ 0.05). Data were analyzed with the IBM SPSS v25.0 software (SPSS Inc. Chicago, IL, USA). Ten replicates per treatment were considered in this experiment.
Results and discussion
Mechanical properties
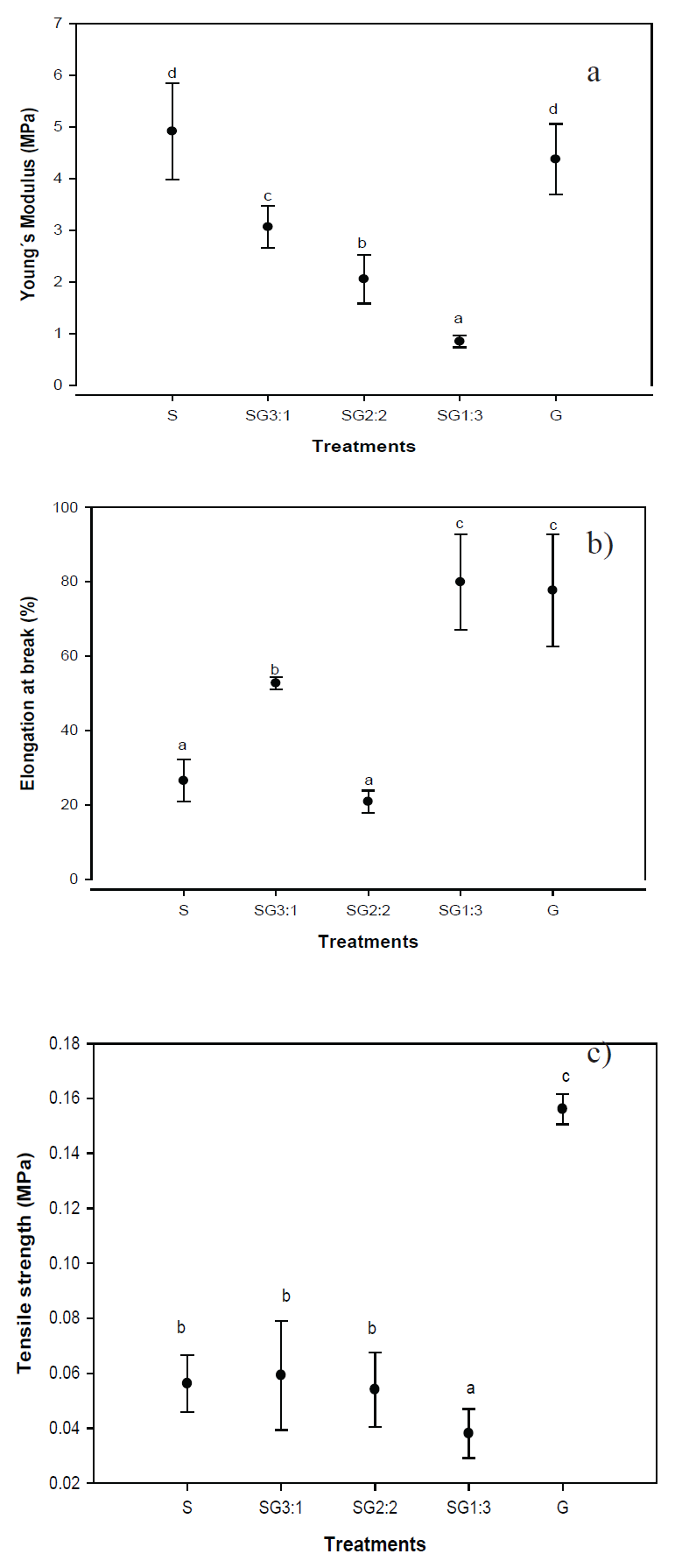
The resistance of films to fracture is an important parameter that describes the structural conformation of films. The mechanical performance of this biomaterial could be of great importance when applied as a protector for food products, as well as pharmaceutical applications. Figure 1 shows that the interaction of the two biopolymers affected the young’s module (E) significantly (P<0.05). The viscoelastic properties of films appeared to decrease as the amount of gelatin increased. Starch films showed 5MPa and the addition of protein decreased E up to 1MPa at the SG 1:3 ratio. These results agree very well with those obtained for previous work, carried out with sago starch and fish gelatin (Al-Hassan and Norziah, 2012). The water/gelatin/starch interactions are mainly produced through hydroxyl groups of starch chains. Also, the presence of small molecules such as water increase the possibility of interaction through hydrogen bonding between starch and gelatin (Tolstoguzov, 1994).
The fracture properties of films represented for the tensile strength and elongation at break demonstrated that the presence of gelatin in films produced brittle continuous networks with large deformation properties. The increment of gelatin in all formulation with starch reduced the elongation at break parameter as a result of the interaction between hydroxyl groups of both starch and gelatin (Al-Hassan and Norziah, 2012). G sample reflected the highest force applied to fracture with 0.15 MPa. These results suggest that there was a better degree of crosslinking in this particular sample. The formation of films in samples with presence of starch was attributed to the entanglement of amorphous chains rather than ordered aggregates or junction points (Kramer, 2009; Wang et al., 2017). The films formulated with starch resulted with limited mechanical properties such as fragile and brittle (Wang et al., 2007). The growing interactions between the hydroxyl groups in glycerol and carboxyl groups of amyloses in starch and gelatin, could increase interfacial interactions between these biopolymers avoiding weak links that create strain in the bio composed matrix.
The elongation at break depends, as tensile strength do, on different factors in mixed system between starch and gelatin which include the hydrogen bonding through their hydroxyl groups. The anionic domains of starch form a network with the cationic domains of gelatin molecules between anionic ones (Fonkwe et al., 2003). G sample and the SG 1:3 mixture showed the highest deformation with 80% compared to the rest of the samples. Previous work carried out in gelatin films in presence of glycerol, have shown that glycerol is compatible with gelatin, and increases significantly its plasticity resulting in higher flexibility and transparency (Al-Hassan and Norziah, 2012; Park et al., 2008).
FT-IR spectroscopy
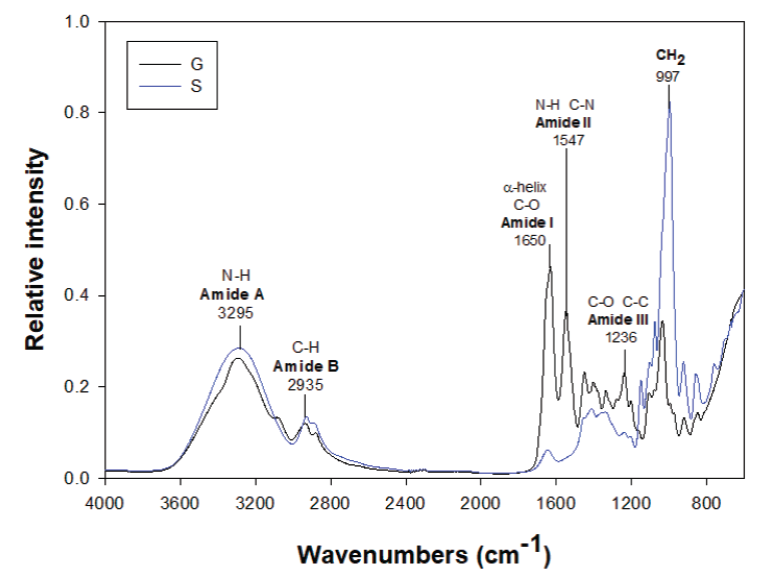
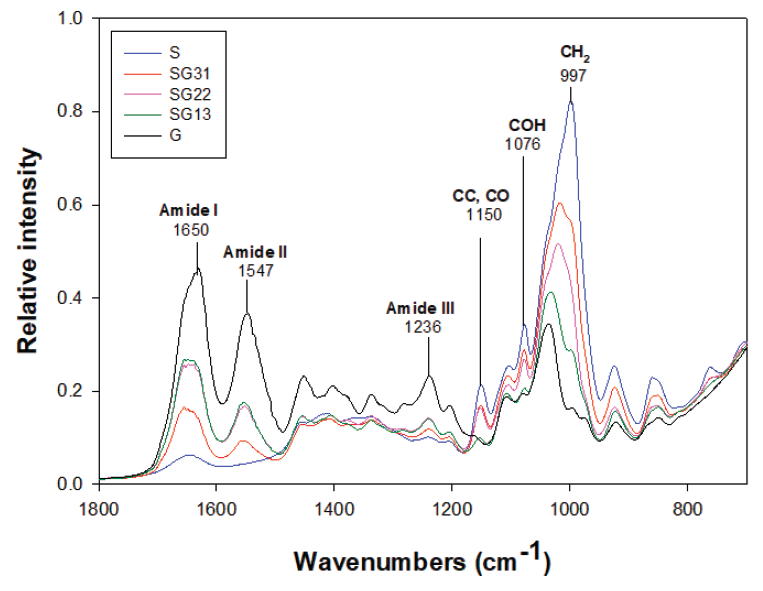
The rheological and mechanical properties of both starch and gelatin depend on their morphology, particularly on their grade of homogeneity and the composition of the continuous and disperse phase (Liu et al., 2014). Figure 2 shows the FTIR transmission spectra of typical gelatin and starch films. The spectral characteristics for gelatin are amide I and II bands which are located approximately at 1650 y 1547 cm-1 respectively. The absorption of amide I was mainly due to the vibration of elongation of the C=O link and amide II peaks resulted from the flexion of the N-H link and elongation of the C-N link. The CO and CC groups were observed at 1236 cm-1. They were related to conformation of crystalline region of starch (Mutungi et al., 2011). The bands of absorption at approximately 1150, 1125 and 1105 cm-1 were due to CO, CC extending to some contributions of COH and related modes with CH2. For starch a series of peaks overlapping located in the region of 1180-953 cm-1 were more intense bands. Also, 3400-3100, 3000-2700 cm-1 bands belong to an extension of vibrations between OH and CH respectively (Muyonga et al., 2004). It was observed that all the bands of absorptions appeared according to the origin of single materials and there were not observed new bands for blends (see Figure 3).
Degree of crystallinity in films
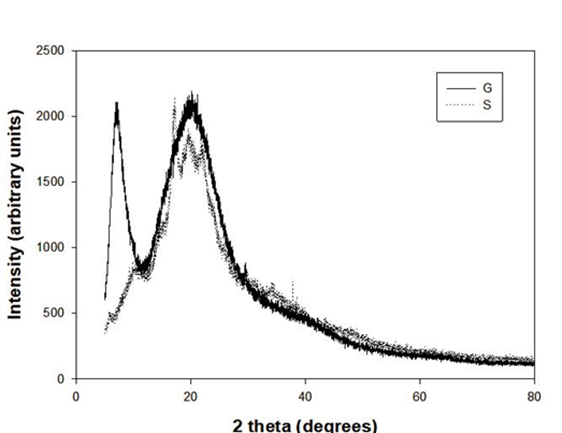
Films presented a pattern of diffraction characteristic of a partially crystalline material. Figure 4 shows the typical diffractograms for G and S sample. G sample showed peaks located in the region around 7° and 20° (Tao et al., 2018). These peaks are associated with diameter and intensity of the triple helix structure reconstructed from collagen (Rivero et al., 2010).
Functional groups of gelatin interfered with the re-assembly of the structure during the film structure formation process (Wang et al., 2017). Previous works on gelatin films (Bigi et al., 2004) have shown that the peak around 7° is related with the diameter of the triple helix and its intensity is associated with the content of the triple helix in films. Peaks presented in starch film at 17°, 20° and 22° were characteristic of crystal proportions that remained in films after heating the sample before preparation of films. It means that thermic energy proportioned at 85°C in the preparation of film was not enough to unchain complete gelatinization of that phase in starch (Gao et al., 2021). However, this thermal treatment unchained a rupture in the granule of starch releasing amylose and amylopectin in the filmogenic solution (Kramer, 2009).
From Table 2, it can be observed that S sample reported significant differences (P < 0.05) with 55.15 % of amorphous content and 44.83% of crystalline content. Comparing these results with G sample, a lower amount of amorphous content (35.03 %) was observed. However, the amorphous content of mixed samples resulted significantly (P < 0.05) higher compared to Gelatin. The SG 2:2 sample showed the higher content with 62.13 %. The higher the amount of starch in films formulation, the greater the amorphous proportion was measured.
Table 2 Degree of Crystallinity in gelatin-starch films. Different letters represent significant differences (P ˂ 0.05). Each value represents the average of 3 replicates.
Tabla 2 Grado de cristalinidad en películas de almidón-gelatina. Diferentes letras representan diferencias significativas (P ˂ 0.05). Cada valor representa el promedio de3 repeticiones.
| Sample | Amorphous content (%) | Crystalline content (%) |
| S | 55.16 ± 2.98 bc | 44.83 ± 2.98 ab |
| SG 3:1 | 46.02 ± 4 ab | 53.97 ± 4 bc |
| SG 2:2 | 62.13 ± 1.39 c | 37.86 ± 1.39 a |
| SG 1:3 | 55.09 ± 1.69b c | 43.90 ± 0.88 ab |
| G | 35.03 ± 8.82 a | 64.96 ± 8.82 c |
During the drying of the film solutions, the molecules followed a tendency to reorganize towards its native structure (Ledward, 1986; Parker and Ring, 2005). The speed of drying in S treatments were higher than the speed of the reorganization of its structure, resulting in a predominance of the amorphous proportion in films. However, the G film reached a partial reorganization to collagen-like structure before the filmogenic solution dried (Aguirre-Álvarez et al., 2011). The films from samples SG 3:1, SG 2:2, SG1:3 and G were produced from gel to solid state with a more ordered structure. This behavior is congruent with the observed data in the determination of crystallinity as the G films presented 64.96 % of crystallinity. The ratio of crystallinity decreased with the presence of starch in the filmogenic solution up to 37.86 % in the SG 2:2 sample. This could be the reason why the sample G resulted more brittle and with higher tensile strength compared to the rest of the samples.
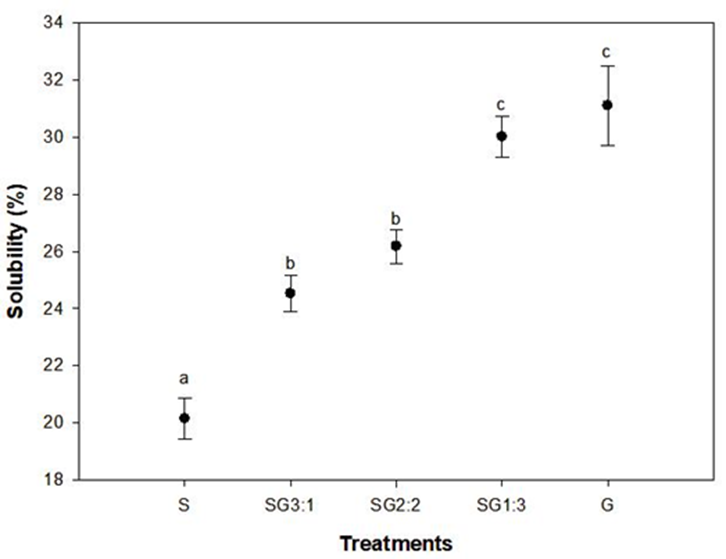
Solubility of films
The solubility of edible films such as starch-gelatin results of greater importance when applied as a packaging material in foods. Figure 5 shows significant differences between treatments (P<0.05). The higher the amount of gelatin, the higher the solubility of films.
The reduction in film solubility could be attributed to the physical interference when starch interacts with gelatin chains entanglement within the film matrix (Guo et al., 2014). The preparation of films involved a thermal treatment of native starch. This process of gelatinization produced very reactive OH groups when exposed to water (Sun et al., 2013). However, blends of starch and gelatin interact each other by means of hydrogen bonds blocking hydroxyl positions capable of association with water (Ahmad and Sarbon, 2021; Flores et al., 2010). The presence of gelatin in the mixtures increased this solubility significantly up to approximately 30% for SG 1:3. This particular sample showed no significant differences (P>0.05) with G treatment prepared only with gelatin and glycerol. These results were lower compared to those reported for films prepared with fish gelatin and chitosan (Fakhreddin Hosseini et al., 2013), chicken skin and tapioca starch (Loo and Sarbon, 2020) and gelatin with potato starch (Aitboulahsen et al., 2020). It is well known that gelatin possesses hydrophilic properties due to some amino acids such as lysine, serine, arginine, hydroxyproline, and aspartic and glutamic acids (Schrieber and Gareis, 2007; Theerawitayaart et al., 2019). These results suggest that addition of starch to gelatin films reduces the gelatin degree of solubility due to electrostatic forces undergoing associative interactions to form a polyelectrolyte complex, which may cause the reduction in water solubility (Fakhreddin Hosseini et al., 2013).
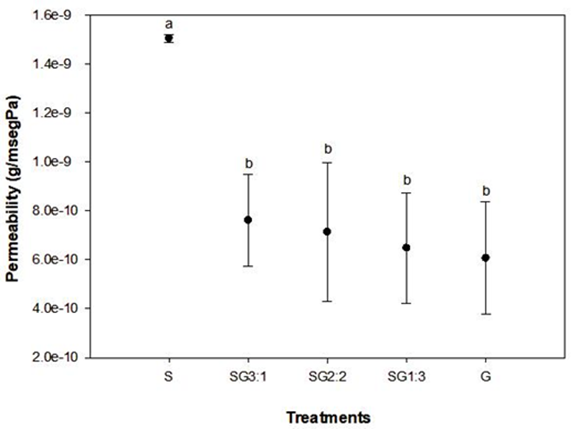
The barrier properties of starch-gelatin films
The water vapor permeability (WVP) is the flux of molecules through a material normalized to a pressure gradient (Tyuftin and Kerry, 2021). WVP of films prepared with starch (S) resulted in greater amount compared to the films prepared with gelatin. Figure 6 shows that the presence of gelatin decreased the WVP no matter what the amount of gelatin was added. Sample S showed high WVP due to its abundance of hydrophilic hydroxyl groups present within amylose concentration of chayotextle starch. This affinity to water molecules promoted the migration of water vapor molecules through the film (Alias and Mhd Sarbon, 2019). The same trend was observed in previous work carried out in films prepared with sago starch-gelatin and glycerol (Al-Hassan and Norziah, 2012). The greater the amount of starch, the higher the WVP was obtained. For treatments in presence of gelatin, it is believed that the starch hydroxyl groups interacted easily with gelatin amino groups via hydrogen bonds to form physically reticulated networks with more compact structure between starch and gelatin, reducing permeation of water molecules into the matrix of films (Ahmad et al., 2015; Wang et al., 2017; Xu et al., 2005). This behavior could be explained in terms of differences in structural properties as the hydrophilic character of the matrix promoted different transport rates of water adsorption capacity in films containing gelatin (Moreno et al., 2016). Previous works have demonstrated that WVP of films depended on several factor such as the ratio between the amorphous and crystalline zones (Garcia et al., 2000), and the greater the crystalline/amorphous ratio, the lower the permeability (Donhowe and Fennema, 1993). The same behavior was observed in films prepared with chayotextle starch and cellulose nanoparticles (Aila-Suárez et al., 2013), the higher the amount of cellulose nanoparticle, the higher the crystallinity of films was obtained, shifting the WVP to lower values.
Conclusion
The characterization of starch-gelatin films was successful by obtaining films with a diversity of characteristics of resistance, solubility and permeable abilities. The films prepared with gelatin resulted more brittle and rigid. However, the addition of starch changed significantly the characteristics of the films presenting lower rigidity and solubility properties. Based on the interaction of starch and gelatin in the mixed systems, it is concluded that structural properties in films can be adapted to the needs and requirements of different industries such as pharmaceutical, biomedical and food engineering. As examples, the following applications of these biomaterials could be mentioned: coatings and packaging of fresh meat, coating of fruits and vegetables, soft gel capsules contained, matrix for the release of drugs, bioactive ingredients, among others.











 nueva página del texto (beta)
nueva página del texto (beta)