Introduction
Inflammation is a physiological process that initiates in response to a bacterial infection or damaged vascularized living tissue (Hassan-Ahmed et al., 2016; Li et al., 2016). Although the fact that the response of this tissue is generally to protect itself, when it is uncontrollable, recurrent, or chronic, it can be associated with diseases such as asthma, obesity, rheumatoid arthritis, among others (Rizzello et al., 2016). The main treatments used for inflammation are steroidal and non-steroidal drugs; however, their use is limited due to their multiple side effects, and therefore, interest is currently emerging for the development of alternative therapies from natural sources, which do not represent risks to health (Sharma et al., 2011; Daliri et al., 2017).
On the other hand, reactive oxygen species (ROS), free radicals, and peroxides can be generated in different metabolic processes or by adverse environmental exposures (Sies and Jones, 2020). Naturally, excess ROS are balanced by the cellular antioxidant system, however, when ROS accumulate beyond the cell’s antioxidant capacity homeostasis is disrupted due to an imbalance between ROS production and the biological detoxification system, which causes oxidative stress (Guo et al., 2014; Torres-Fuentes et al., 2015). This has as a consequence of DNA, proteins, and lipids modifications, in addition to playing a significant role in the appearance of diseases such as cancer, arteriosclerosis, cardiovascular diseases, diabetes mellitus, neurological disorders, Alzheimer’s disease, Parkinson’s disease, and even chronic inflammation (Guo et al., 2014; Elshafei, 2020).
Therefore, at present is important to search for endogenous antioxidant substances that act as direct scavengers of free radicals, or as chelating agents of metal ions that catalyze the generation of radical species, which could delay the progress of many chronic diseases or attenuate chronic inflammation (Zhang et al., 2018; Rizzo, 2020).
Proteins and their peptides obtained from legumes have shown ABTS and DPPH radical inhibitory activity, reducing power, and metal chelating capacity (Samaei et al., 2020; Wen et al., 2020). Likewise, peptides that inhibit the produc-tion of pro-inflammatory mediators such as cyclooxygenase (COX-2), inducible nitric oxide synthase (iNOS), prostaglandin (PG) E2 and D2, nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), and interleukins (IL)-1β and IL-6 in RAW 264.7 macrophages stimulated by E. coli lipopolysaccharides (LPS), have been reported (Millán-Linares et al., 2015; González-Montoya et al. 2018).
Chickpea (Cicer arietinum L.) is a rich source of protein (17-23 %), in addition to containing carbohydrates (70 %), lipids (4-10 %), vitamins (B group), and minerals like potassium, phosphorus, magnesium, and calcium (Ercan and Neheir, 2016; Mieszkowska and Marzec, 2016). Chickpea proteins have high bioavailability, and due to their amino acid profile after hydrolysis, peptides with hypolipidemic, antioxidant and antimicrobial activity, among others, have been identified (Ghribi et al., 2015b; Shi et al., 2019; Wali et al., 2020). However, bioactive peptides isolated from enzymatically hydrolyzed chickpea proteins that show anti-inflammatory activity have not been reported. Therefore, the aim was to evaluate the anti-inflammatory and antioxidant effect of chickpea (Cicer arietinum L.) proteins hydrolysate fractions by in vitro evaluation of inflammatory mediators in LPS-activated murine peritoneal macrophages and on the inhibition of free radicals, so that, possibly the chickpea proteins hydrolysate fractions can be used to isolate bioactive peptides with anti-inflammatory activity.
Materials and methods
Material
Kabuli chickpea (Cicer arietinum L.) Valle Verde brand lot L7277G1B was obtained from a local supermarket. Murine peritoneal macrophages were isolated from ICR mice, fetal bovine serum (S1480) was purchased from Biowest (Riverside, MO, USA), antibiotic-antifungal (10,000 μg/mL streptomycin, 10, 000 µg/mL penicillin, and 25 µg/mL amphotericin B) (A-07) was purchased from Vitro S. A. (Granada, Spain), Alcalase 2.4 L (EC3.4.21.62) and Flavourzyme (EC3.4.11. 1) were purchased from Novo Nordisk (Bagsvaerd, Denmark), Pepsin from porcine gastric mucosa (EC 3.4.23.1), Pancreatin from porcine pancreas (EC 232.468. 9), 2-diphenyl-1-picrylhydrazyl (DPPH), 2-20-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), RPMI-1640 medium (GIBCO 31800-014), lipopo-lysaccharide from E. coli O55:B5 (L2880), TNF-α mouse ELISA Kit (RAB0477) and IL-1β mouse ELISA Kit (RAB0274) were purchased from Sigma-Aldrich Company (St. Louis, MO, USA).
Preparation of chickpea defatted flour
Chickpeas were selected and ground (High speed electric pulverizer mill, XHH-39-WJY, EUA) and passed through a 35 mesh sieve, then the fat was removed from the flour to obtain a higher protein yield in the concentrate, for which it was resuspended in hexane (1:4 [w/v], flour: hexane) and placed in stirring (500 rpm) for 24 h at 4°C, then the solvent was removed by filtration and the powder was left to dry at room temperature for 24 h and finally stored at 4°C until further use (Mondor et al., 2009).
Preparation of chickpea proteins concentrate and extrac-tion of phenolic compounds
Chickpea proteins concentrate was obtained by isoelectric precipitation according to Ghribi et al. (2015a). Briefly, the defatted flour was mixed with distilled water in a proportion 1:10 w/v (flour: water), the pH was adjusted to 9.0 using 40 % NaOH and kept for 2 h at 25°C with mechanical stirring (500 rpm), maintaining the pH throughout the process. Subsequently, the mixture was centrifuged (5000 g/4°C/20 min). The supernatant obtained was adjusted to pH 4.5 (isoelectric point) with 1N HCl and kept for 30 min. Finally, it was centrifuged at 5000 g/4°C/20 min (Hermle, Z446K, Wehingen, Germany). The precipitate was lyophilized (Labconco, FreeZone 2.5, Kansas City, MO) at -51°C and 0.3 mBar.
Phenolic compounds that could interfere in the analyzes were removed and determined following the methodology of Carrasco-Castilla et al. (2012). Briefly, protein concentrate (1:10 w/v) was kept under stirring (500 rpm/4°C for 30 min) in acetone 75 % and separated by decantation 5 times. Finally, the protein concentrate was kept at room temperature until the complete removal of the solvent. The determination of total phenols was determined according to the Folin-Ciocalteau method. The results were expressed in mg GAE/g dry basis.
Enzymatic hydrolysis of chickpea proteins concéntrate
Hydrolysis was performed with two different systems: Alcalase®-Flavourzyme® (Alcalase 2.4L EC 3.4.21.62; Flavourzyme EC 3.4.11.1) and Pepsin®-Pancreatin® (Pepsin EC 3.4.23.1; PP-77163, from porcine gastric mucosa, Pancreatin P-1750, porcine pancreas, 4xUSP). Briefly, the first enzyme of each system was added at time 0 and the second enzyme 60 min later. The hydrolysis parameters for each system were: protein concentration 5 % (w/v) for both systems, 50°C for Alcalase-Flavourzyme and 37°C for Pepsine -Pancreatine. For Alcalase, enzyme/substrate ratio 0.3 AU/g and pH 8.0, Flavourzyme enzyme/substrate ratio 50 LAPU/g and pH 7.0, Pepsin 4 USP/g and pH 2.0, Pancreatin 4 USP/g and pH 7.5. Aliquots of the hydrolysates were taken at 0, 30, 60, 90, 120, 150, 180 min. Then, the enzymes were inactivated in a water bath at 80°C for 10 min, centrifuged at 5000 x g for 20 min to separate the soluble fraction, which was lyophilized at -51°C and 0.3 mBar (Ghiribi et al., 2015b).
Degree of hydrolysis
The degree of hydrolysis (DH) was determined accor-ding to the o-phthaldehyde (OPA) method described by Niel-sen et al. (2001). Briefly, a 10 µL aliquot (sample or standard) was placed in a 96-well plate, mixed with 200 µL of OPA rea-gent for 5 s and incubated for 2 min. Finally, the absorbance was measured at 340 nm in a microplate reader (ThermoLab Systems, Multiskan Spectrum, MA, USA). L-serine was used as standard. The calculation of the degree of hydrolysis was carried out by applying equations 1, 2, and 3.
Where:
DH = Degree of hydrolysis
H = Number of peptide bonds broken
htot = Total number of bonds in the substrate under study
Where:
α and β are constants, 1.0 and 0.40, respectively. V = Sample volume in liters
X = Sample weight in grams
P = Sample content expressed as a percentage DF = Dilution factor
Ultrafiltration fractionation of chickpea proteins hydro-lysate
Chickpea proteins hydrolysate that showed the highest degree of hydrolysis was fractionated in an ultrafiltration system (Millipore, Amicon 2000, Darmstadt, Germany) through ultrafiltration membranes cut-off of 10, 5, 3, and 1 kDa. The fractions were recovered and kept frozen at -21°C for further use (Millán-Linares et al., 2015). The protein content of the obtained fractions was determined with the bicinchoninic acid (BCA) method using the commercial kit adapted for microplate.
Determination of anti-inflammatory activity Cell culture
Isolation of murine peritoneal macrophages was performed according to the methodology described by Layoun et al. (2015). Female mice of the ICR strain were used for macrophage isolation, maintained in an adaptation period of 7 days in the biotherium conditions (22-24°C, humidity 50-55 % with a 12 h light/dark cycle). Subsequently, sterile thioglycollate was administered into the peritoneal cavity (10 % w/v), and possible behavioral changes, water, food consumption, and weight loss were monitored until sacrifice.
After 72 h, animals were sacrificed, without damaging the peritoneal cavity, and macrophages were extracted by cold 1X phosphate-buffered saline (PBS) washes. The suspension was centrifuged at 5 min/1200 g/4°C, and the cell pellet obtained was resuspended in RPMI 1640 medium (GIBCO 31800-014) supplemented with 10 % fetal bovine serum (FBS). For macrophage adherence, 1 mL (1x106) of the cell suspension was seeded in 24-well plates. The plate was incubated for 2 h/37°C/5 % CO2, and subsequently, the medium was removed, and 1 mL of RPMI 1640 medium supplemented with 5 % FBS was added to the control well and the treatments prepared in 5 % medium in the corresponding wells (5, 2.5, 1.25, and 0.625 mg/mL), and incubated 30 min/37°C, 5 % CO2. Then, 10 µL of LPS (5 µg/mL) were added to each well, and the plate was incubated for 18 h/37°C, under 5 % CO2 (Soudi et al., 2013). At the end of this period, the supernatants were collected and kept frozen (-21°C) until the inflammatory mediators were determined.
Cell viability
Cell viability of murine peritoneal macrophages was determined with the sulforhodamine B (SRB) method according to Orellana and Kasinki (2016) in a microplate. Briefly, 100 µL of 1x106 cells/mL in RPMI 1640 medium with 10 % FSB were placed in a 96-well plate and incubated for 2 h/37°C/5 % CO2. Then, the medium was discarded and 100 µL of the treatments to be evaluated were added at 4 different concentrations (5, 2.5, 1.25, and 0.625 mg/mL), all diluted in RPMI 1640 medium at 5 % FSB, and the plate was incubated (18 h/37°C/5 % CO2). After this time, 50 µL of 50 % trichloroacetic acid were added and plates incubated for 1 h at 4°C; the supernatant was discarded and the plate was washed 5 times with distilled water. Once dry, 100 µL of 0.4 % SRB were added and incubated for 30 min at room temperature in the dark. Finally, the supernatant was removed, washed 5 times with 1 % acetic acid, dried and 100 µL of 10 mM buffer tris were added. It was read at an absorbance of 492 nm. The percentage of viable cells was calculated with respect to control cells (untreated cells), considering viable cells (%) with respect to control cells (untreated cells, 100 %).
Nitric oxide (NO)
Nitrate was measured as an indicator of NO production after 18 h of induction with LPS and treatments. Briefly, in a 96-well plate, 100 µL of cell culture supernatant were placed, then 100 µL of Griess reagent (2 % sulfonyl amide and 0.1 % N-(1-naphthyl)-ethylenediamine) were added. Finally, the plate was incubated for 5 min and the absorbance was read at 540 nm. The amount of NO (µM) was calculated using a NaNO2 standard curve (0-200 µM), y = 0.0075x + 0.0602 (r2 = 0.999), where y = absorbance and x = nitrite concentration (µM) (Avdagić et al., 2013).
Determination of TNF-α and IL-1β
According to the manufacturer’s instructions, the TNF-α concentration in cell culture supernatants was obtained using a commercial Kit (RAB0477, Sigma-Aldrich). The amount of TNF-α (pg/mL) was calculated using a TNF-α standard curve (0-3000 pg/mL), y = 0.0011x - 0.085 (r2 = 0.9851), where y = absorbance and x = TNF-α concentration (pg/mL). The amount of IL-1β (RAB0274, Sigma-Aldrich) was calculated using IL-1β standard curve (0-2000 pg/mL), y = 0.0013x + 0.0817 (r2 = 0.998), where y = absorbance and x = IL-1β concentration (pg/mL).
Determination of antioxidant activity
The ABTS radical-scavenging activity was determined by the method of Dinis et al. (1994). The absorbance was measured at 734 nm against a methanol blank. The results were expressed as % inhibition. The DPPH radical scavenging capacity was determined according to Brand-Williams et al. (1995) measuring the absorbance at 517 nm against a methanol blank. The results were expressed as % inhibition. The reducing power was determined by the method of Oyaizu (1986). The absorbance was measured at 700 nm. In this method, a higher absorbance of the reaction mixture indicates a higher reducing power. The chelation capacity Cu2+ was determined according to Dinis et al. (1994). The absorbance was measured at 632 nm. The results were expressed as % inhibition.
Results and discussion
Degree of chickpea proteins hydrolysis
The hydrolysis of chickpea proteins was carried out from the concentrate with a protein content of 79.92 ± 1.77 %. The kinetics of the proteolytic reaction with the two-enzyme systems Alcalase-Flavourzyme (A-F) and Pepsin-Pancreatin (P-P) is presented in Figure 1.
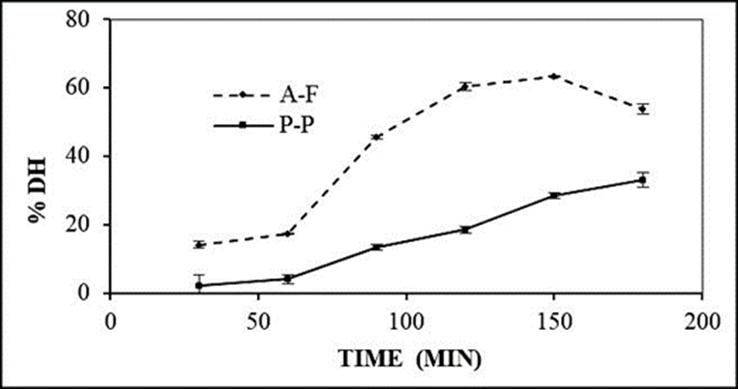
The P-P enzyme system showed a degree of hydrolysis of 33.18 % at 180 min. This result agrees with that reported by Torres-Fuentes et al. (2011)) who obtained a degree of hy-drolysis of 32 %. The degree of hydrolysis obtained from the A-F system was 63.31 % at 150 min, similar to that reported by Yust et al. (2012) with a degree of hydrolysis of 65 % for chickpea protein with a sequential system of Alcalase®-Flavourzyme® for 150 min. The maximum degree of hydrolysis (63.31 %) was reached with the A-F system at a time of 150 min since at 180 min there was a decrease (53.81 %). Torres-Fuentes et al. (2011) reported a similar trend in the degree of hydrolysis generated by the Pepsin®-Pancreatin® enzymatic system, they obtained 31.5 % after 325 min, however, at the end of hydrolysis (360 min) they reported a 27 % for the hydrolyzed chickpea protein. This was probably due to the plastein reaction, which is a series of reactions catalyzed by proteases that condense peptides, so that peptide fragments of hydrolysates can join together forming polypeptides, resulting in high molecular weight proteins (Torres-Fuentes et al., 2011; Udenigwe and Rajendran, 2016).
On the other hand, several studies report that the use of enzymes individually generates a degree of hydrolysis <30 %. Ghribi et al. (2015b) obtained a 14.67 % hydrolysis from Al-calase® after 120 min, while Torres-Fuentes et al. (2011) reported 16.3 % with the enzyme Pancreatin® at 180 min. Therefore, using two enzymes for protein hydrolysis is more effective in producing extensive hydrolysates (>50 %) (Yust et al., 2012). The use of the A-F enzyme system produced an extensive hydrolysate due to the Alcalase® enzyme, which, being an endopeptidase, influences the tertiary and quaternary conformations of the protein by breaking the peptide bonds (preferably hydrophobic amino acids) within the individual proteins or aggregates to produce small peptides and thus perform a pre-digestion. This, in turn, increases the number of N-terminal sites, thereby facilitating hydrolysis with the Flavourzyme® enzyme, which is a complex of proteases from Aspergillus oryzae with endopeptidase and exopeptidase activity, resulting in high degrees of hydrolysis within 150 min (Ghribi et al., 2015b; Nasri, 2016; Tacias-Pascacio et al., 2020).
The degree of hydrolysis depends on the conditions used, the nature of the enzyme and its enzyme-substrate concentration, hydrolysis time, temperature, and pH. Likewise, these parameters affect the peptide structure in terms of size, composition, and amino acid sequence, which determines the bioactivity of the peptides. Therefore, high levels of proteolysis are desirable (>50 %) since extensive hydrolysate is necessary to obtain various bioactive peptides for medical or dietary applications (Agyei et al., 2016; Nasri, 2016).
On the other hand, studies have reported that the antioxidant activity presented by peptides obtained from hydrolyzed chickpea protein is higher in peptides with low molecular weight presenting sequences like ALEPDHR, TETWNPNHPEL, FVPH, and SAEHGSLH (Torres-Fuentes et al., 2011; Torres-Fuentes et al., 2015), which is related to the time used in hydrolysis. Megías et al. (2007) reported peptides from chickpea hydrolyzed protein with Alcalase®-Flavourzyme® enzymes for 10 min and showed low chelating activity, while peptides purified from 100 min hydrolysates with the same enzymes showed high chelating activity. This indicates that a longer hydrolysis time can generate peptides with lower molecular weights that presents major antioxidant activity.
Enzymatic hydrolysis is the most preferred method over others since chemical hydrolysis has the disadvantage of destroying some amino acids and has a difficult reproducibility since the broken bonds are not specific. Likewise, microbial hydrolysis is not suitable as it produces low-yield peptides that are sometimes used as a source for the growth of the same microorganism (Nasri, 2016).
Based on the above, hydrolysis performed with the Alcalase®-Flavourzyme® system for 150 min is the most suitable method to obtain extensive chickpea proteins concentrate hydrolysates with a degree of hydrolysis greater than 50 %, so that the concentrate can be a source of low molecular weight bioactive peptides that can present biological activities such as anti-inflammatory and antioxidant.
Cell viability
Although bioactive peptides possess biological activities, there is a possibility of a cytotoxic effect, so it is necessary to evaluate the harmful effects (Sarmadi and Ismail, 2010). The SRB method is characterized as fast, sensitive and inexpensive, so it was used to determine the cytotoxic effect of the 4 fractions obtained from hydrolyzed chickpea proteins at 4 different concentrations (5, 2.5, 1.25, and 0.625 mg/mL).
Figure 2 shows that the F≤1 kDa fraction generated the lowest viability (76.3 %) at a concentration of 5 mg/mL, however, this fraction did not show a significant difference in any of its concentrations. In contrast, the rest of the fractions (F5-10 kDa, F3-5 kDa, and F1-3 kDa) did not decrease viability at any of the concentrations used. Therefore, for NO, TNF-α, and IL-1β determinations, only the 5-10 kDa, F3-5 kDa, and F1-3 kDa fractions were evaluated. F≤1 kDa fraction was not evaluated since it showed a decrease in the viability of murine peritoneal macrophages.

Figure 2 Cell viability of the 4 fractions obtained from the hydrolyzed chickpea protein at different concentrations (5, 2.5, 1.25, and 0.625 mg/mL). Results represent the mean of three independent determinations ± SD. Values that do not share the same lowercase letter within the same fraction are significantly different (p<0.05).
Figura 2 Viabilidad celular de las 4 fracciones obtenidas de la proteína hidrolizada de garbanzo en sus diferentes concentraciones (5, 2.5, 1.25 y 0.625 mg/mL). Los resultados representan la media de tres determinaciones independientes ± DE. Los valores que no comparten la misma letra minúscula dentro de la misma fracción son significativamente diferentes (p <0.05).
Determination of NO
Nitrate was measured as an indicator of NO production since it plays a crucial role in the inflammatory process, so its inhibition may help regulate inflammation (Ndiaye et al., 2012). As shown in Figure 3, LPS stimulated NO synthesis compared to the control group (macrophages without LPS and without treatment). The three fractions obtained from chickpea proteins hydrolysate showed NO production inhibition. The F3-5 kDa fraction showed the lowest amount of NO (15.41 µM) at a 5 mg/mL concentration, followed by the F1-3 kDa (22 µM) and F5-10 kDa (18.81 µM) fractions. The above indicates that the 3-5 kDa fraction presented the highest percentage of NO inhibition (76 %) with respect to the cells stimulated with LPS, at a 5 mg/mL concentration. These results are similar to those reported by López-Barrios et al. (2016), of a 73 % NO inhibition from a black bean germinated protein hydrolysate.
Figure 3 Effect of the three peptide fractions obtained from the hydro - lyzed chickpea protein on the inhibition of NO in LPS-activated murine peritoneal macrophages. Results represent the mean of three independent determinations ± SD. Values that do not share the same lowercase letter within the same fraction are significantly different (p<0.05). Values that do not share the same capital letter between fractions are significantly different (p<0.05).

#Representa diferencia significativa (p <0.05) con respecto al grupo control.
*Representa diferencia significativa (p <0.05) con respecto al LPS.
#Represents significant difference (p <0.05) with respect to control group.
* Represents significant difference (p <0.05) with respect to LPS group.
Figura 3 Efecto de las tres fracciones peptídicas obtenidas a partir de la proteína de garbanzo hidrolizada sobre la inhibición del NO en macrófa-gos peritoneales murinos activados por LPS. Los resultados representan la media de tres determinaciones independientes ± DE. Los valores que no comparten la misma letra minúscula dentro de la misma fracción son significativamente diferentes (p <0.05). Los valores que no comparten la misma letra mayúscula entre fracciones son significativamente diferentes (p <0.05).
The reduction in the inhibition of NO synthesis observed could be due to the ability of the peptides contained in the chickpea proteins hydrolysate fractions to inhibit the expression of the inducible nitric oxide synthetase enzyme (González-Montoya et al., 2018), which is responsible for oxidizing the amino acid L-arginine into L-citrulline and thus the expression of NO, which is a mediator of inflammation mainly synthesized by macrophages (Huang et al., 2016). However, when this is found at high concentrations is considered cytotoxic, leading to molecular damage that can generate a continuous state of inflammation which is related to several diseases including chronic inflammatory ones (Ndiaye et al., 2012), therefore, inhibiting NO synthesis can help to control the acute or chronic inflammatory response.
On the other hand, it has been reported that peptides obtained from germinated and hydrolyzed chickpea proteins have molecular weights between 1.1-2.3 kDa, and sequences of 10-22 amino acids have shown inhibition of NO production in RAW 264.7 macrophages activated by LPS (Milán-Noris et al., 2018). However, molecular weight is not the only factor that is related to the biological activities presented by the peptides obtained from legume proteins (Nasri, 2016), so the NO synthesis inhibitory activity shown by the peptides present in the 3-5 kDa proteins hydrolysate fraction in the present study, can be attributed to the amino acids present, their sequence or their charge.
Determination of TNF-α and IL-1β
IL-1β together with TNF-α are considered the main mediators of the biological response to LPS (García-Lafuente et al., 2014). Thus, inhibition of the expression of these inflammatory mediators may be a strategy to control the inflammatory response in chronic conditions, and thus be able to contribute to the treatment of chronic inflammatory diseases. After stimulation with LPS, TNF-α production was determined in the different groups. The F3-5 kDa fraction at 5 mg/mL, induced the lowest amount (179.5 pg/mL) of TNF-α followed by F5-10 kDa (185.69 pg/mL) and F1-3 kDa (192.27 pg/mL). Therefore, the F3-5 kDa fraction presented the highest percentage of inhibition (93 %) with respect to the LPS group (Figure 4). These results are similar to those reported by Vernaza et al. (2012) for a soy protein hydrolysate that presented a 98 % inhibition of TNF-α.
Figure 4 Effect of peptide fractions (F5-10, F3-5 and F1-3 kDa) obtained from the hydrolyzed chickpea protein (5, 2.5, and 1.25 mg/mL), on the inhibition of TNF-α in LPS-activated murine peritoneal macrophages. Results represent the mean of three independent determinations ± SD. Values that do not share the same lowercase letter within the same fraction are significantly different (p<0.05). Values that do not share the same capital letter between fractions are significantly different (p<0.05).

#Representa diferencia significativa (p <0.05) con respecto al grupo control.
*Representa diferencia significativa (p <0.05) con respecto al LPS.
#Represents significant difference (p <0.05) with respect to control group.
* Represents significant difference (p <0.05) with respect to LPS group.
Figura 4 Efecto de las fracciones peptídicas (F5-10, F3-5 y F1-3 kDa) obtenidas a partir de la proteína hidrolizada de garbanzo (5, 2.5 y 1.25 mg/mL) sobre la inhibición de TNF-α en macrófagos peritoneales murinos activados por LPS. Los resultados representan la media de tres determinaciones independientes ± DE. Los valores que no comparten la misma letra minúscula dentro de la misma fracción son significativamente diferentes (p <0.05). Los valores que no comparten la misma letra mayúscula entre fracciones son significativamente diferentes (p <0.05).
n the case of IL-1β (Figure 5), LPS stimulated the production of IL-1β compared to the control. The 3 fractions presented inhibition of this interleukin, however, F1-3 kDa and F3-5 kDa fractions showed the highest inhibition (87 and 90 %) with respect to LPS-stimulated cells. These results are higher than those reported by Ndiaye et al. (2012) who obtained 80 % inhibition of IL-1β synthesis in LPS-activated macrophages with a pea protein hydrolysate.Studies have reported that, anti-inflammatory peptides have a wide range of molecular weights (0.3-14 kDa) (Dia et al., 2009; Millán-Linares et al., 2015; Ndiaye et al., 2012). Likewise, peptides have been found to be rich in glutamine and serine from germinated and hydrolyzed soybean and chickpea proteins which presented anti-inflammatory activity (González-Montoya et al., 2018; Milán-Noris et al., 2018). Although the possible relationship of the amino acids present in peptides with their anti-inflammatory activity has not yet been defined, some studies have reported peptides that inhibit the action of COX-2, iNOS enzymes and other inflammatory mediators such as PGE2, CCL2, among others (Milán-Noris et al., 2018; Millán-Linares et al., 2015; Oseguera-Toledo et al., 2011). In the present study, F3-5 kDa fraction presented the highest inhibition in the synthesis of cytokines TNF-α and IL -1β, which are produced in various cells including macrophages and are expressed by the action of di-fferent stimuli such as LPS (Montoya-Rodríguez et al., 2014). However, the mechanisms through which the chickpea proteins hydrolysate fractions act by inhibiting the produc-tion of pro -inflammatory mediators (IL-1β and TNF-α) are still unknown. Nevertheless, studies carried out using proteins from legumes such as bean and soybean have reported the inhibition of inflammation through the suppression of the NF-ĸB pathway (Dia et al., 2009; Oseguera-Toledo et al., 2011). Therefore, the chickpea proteins hydrolysate fractions evaluated in the present study possibly carry out their function in a similar way.
Figure 5 Effect of the peptide fractions (F5-10, F3-5, and F1-3 kDa) obtained from the hydrolyzed chickpea protein (5, 2.5, and 1.25 mg/mL), on the inhibition of IL-1β in LPS-activated murine peritoneal macrophages. Results represent the mean of three independent determinations ± SD. Values that do not share the same lowercase letter within the same fraction are significantly different (p<0.05). Values that do not share the same capital letter between fractions are significantly different (p<0.05).

#Representa diferencia significativa (p <0.05) con respecto al grupo control.
*Representa diferencia significativa (p <0.05) con respecto al LPS.
#Represents significant difference (p <0.05) with respect to control group.
* Represents significant difference (p <0.05) with respect to LPS group.
Figura 5 Efecto de las fracciones peptídicas (F5-10, F3-5 y F1-3 kDa) obtenidas a partir de la proteína hidrolizada de garbanzo (5, 2.5 y 1.25 mg/mL) en la inhibición de IL-1β en macrófagos peritoneales murinos activados con LPS. Los resultados representan la media de tres determinaciones independientes ± DE. Los valores que no comparten la misma letra minúscula dentro de la misma fracción son significativamente diferentes (p <0.05). Los valores que no comparten la misma letra mayúscula entre fracciones son significativamente diferentes (p <0.05).
Determination of the antioxidant activity of chickpea protein hydrolysate fractions
During inflammation, cells such as macrophages and neutrophils generate ROS to help destroy foreign agents, however, although these play an important role in immune defense, they are highly unstable and react rapidly with other groups and damage other biological molecules such as DNA, proteins, and lipids, resulting in cellular or tissue injury (Chen et al., 2018). In addition, excessive ROS generation has been linked to chronic inflammation, so the use of antioxidants, such as bioactive peptides with these properties, could help to neutralize these reactive oxygen species (Zhang et al., 2011). Due to the various antioxidant action reported for bioactive peptides, several methods were used to have a broader view on the antioxidant potential, for this purpose four methods with different mechanisms of action were applied.
The fractions with the highest ABTS•+ radical inhibition were F1-3 kDa and F3-5 kDa (78 and 75 %) followed by F≤1 kDa and F5-1 kDa (Table 1). These results agree with that reported by Ngoh and Gan (2016) who obtained a peptide fraction from beans with molecular weight <3 kDa , which showed the highest percentage of ABTS•+ radical inhibition compared to other fractions with higher molecular weight. Likewise, the results show that the four fractions presented greater ABTS•+ radical inhibition compared to the chickpea protein concentrate with (CCF) and without phenolics (CSF), similar to that reported by Arcan and Yemenicioglu (2010) who found that the chickpea protein hydrolysate has greater inhibition of this radical (1.9 times more) compared to the chickpea protein extract. The above may be due to abundant hydrophobic and hydrophilic amino acid residues present in peptides that are part of the peptide fractions with molecular weights <3 kDa obtained by ultrafiltration (Ngoh and Gan, 2016).
Table 1 Antioxidant capacity of chickpea proteins concentrate and fractions obtained from enzymatic hydrolysis.
Cuadro 1 Capacidad antioxidante del concentrado proteico de garbanzo y las fracciones obtenidas por hidrólisis enzimática.
| Sample | ABTS•+% Inhibition | DPPH•+% Inhibition | Reducing power Fe3+ Abs (700 nm) | Chelating activity Cu2+ % Inhibition |
|---|---|---|---|---|
| CCF | 22.27 ± 0.41c | 6.42 ± 0.35c | 0.217 ± 0.01d | 2.48 ± 1.28e |
| CSF | 10.12 ± 0.08d | 7.29 ± 0.32ab | 0.172 ± 0.02d | 2.42 ± 1.37e |
| Fractions (kDa) | ||||
| F5-10 | 64.50 ± 2.80b | 5.22 ± 0.21d | 0.369 ± 0.04c | 40.75 ± 0.85d |
| F3-5 | 74.80 ± 1.24a | 6.51 ± 0.37bc | 0.437 ± 0.03b | 43.05 ± 0.20c |
| F1-3 | 78.00 ± 0.28a | 7.76 ± 0.28a | 0.508 ± 0.01a | 48.49 ± 0.60b |
| F<1 | 67.80 ± 1.26b | 6.88 ± 0.21bc | 0.457 ± 0.03ab | 52.60 ± 0.44a |
CCF = Concentrate with phenolics, CSF = Concentrate without phenolics. Values represent means ± SD. Values that do not share the same letter per column are significantly different (p≤0.05). * Fractions (5 mg/mL) were obtained from CSF.
The fraction with the highest DPPH•+ radical inhibition percentage was F1-3 kDa (7.76 %) followed by F<1 kDa, F3-5 kDa, and F5-10 kDa. These results are similar to those obtained by Yu et al. (2018) who reported the highest DPPH•+ inhibition for the fraction with molecular weights between 1-3 kDa obtained from hydrolyzed soy protein isolate. These authors reported the same behavior in terms of the order of radical inhibition percentage by the four fractions (F1-3 kDa, F <1 kDa, F3-5 kDa, F5-10 kDa). The results obtained are similar to those reported for peptide fractions with molecular weight <3 kDa obtained from hydrolyzed soybean and mung bean protein, which showed the highest percentage of DPPH•+ radical inhibition (Zhang et al., 2018; Xie et al., 2019).
On the other hand, studies have reported peptides obtained from hydrolyzed chickpea protein with very low molecular weights (0.3, 0.4, 0.7, and 1.1 kDa) and sequences ranging from 3-10 amino acid residues, which exhibit DPPH•+ radical inhibition (Zhang et al., 2011; Ghribi et al., 2015b). However, the antioxidant activity is not only attributed to the molecular weight presented by the peptides, since this also seems to be strongly related to the amino acid composition and sequence, conformation, and hydrophobicity. Hydrophobic amino acids such as Phe, Ala, Pro, Val, Gly, and Ile and non-hydrophobic amino acids such as His, Tyr, Gln, Arg, Asp, and Ser have been found in peptides obtained from hydrolyzed chickpea protein which show DPPH•+ radical inhibition capacity, being His and Arg found in almost all the sequences of these peptides; likewise, Asp, Gln, Arg, Pro, Gly, and Ile are terminal amino acids in these peptides (Zhang et al., 2011; Ghribi et al., 2015b; Rizzello et al., 2016). The antioxidant activity of these peptides is attributed in the case of aromatic amino acids to the fact that they convert free radicals into stable molecules by donating electrons, while these amino acids maintain their stability through their resonance struc-ture; similarly, in the case of Asp, is due to the carboxyl group that it presents in its side chains, and for hydrophobic amino acids such as Gly to which is an H+ donor (Ghribi et al., 2015b). So, the hydrolysis of chickpea proteins possibly generated peptides that could react with free radicals to convert them into more stable products and prevent their chain reaction.
The Fe3+ ion reducing power is used to evaluate the ability of an antioxidant to donate an electron to a free radical and convert it into a more stable component (Li et al., 2008). The fraction with the highest reducing power was F1-3 kDa (0.508Abs700nm), followed by F<1 kDa, F3-5 kDa, and F5-10 kDa. These results are similar to those reported by Zhang et al. (2018) who obtained fractions with molecular weight <3 kDa from hydrolyzed mung bean and soybean protein, respectively, which presented the highest reducing power compared to other fractions with higher molecular weight.
The increase in absorbance of the reaction mixture (hydrolysate fraction) is related to the increase in reducing power, furthermore, it is observed (Table 1) that the four fractions presented higher reducing power compared to CCF and CSF. These results are similar to those obtained by Yust et al. (2012) who reported that chickpea protein hydrolysate presented better reducing power (2.16 times more) compared to chickpea protein concentrate.
On the other hand, Torres-Fuentes et al. (2015) reported peptides that presented Fe3+ reducing power, obtained from hydrolyzed chickpea protein with molecular weights between 0.4-1.3 kDa and constituted by 4-11 amino acid residues including Ala, Leu, Glu, Pro, Asp, His, Arg, Thr, Tyr, Asn, Phe, Val, Ser, and Gly, being Pro, Glu and His the most frequently appearing ones; besides, Ala, Arg, Thr, Leu, Phe and His are terminal amino acids in these peptides. Thus, the fractions evaluated possibly contain some peptides that have amino acids that present donor electrons and can react with free radicals to convert them into more stable products and terminate the radical chain reaction (Li et al., 2008).
Transition metal ions such as Cu2+ and Fe2+ can catalyze the generation of reactive oxygen species through Fenton reactions, resulting in lipid peroxidation and DNA damage (Real-Hernández and González de Mejia, 2019). Likewise, it can catalyze the Haber-Weiss reaction and induce the formation of superoxide anions to form more dangerous hydroxyl radicals, which react with adjacent biomolecules to cause severe tissue damage (Adegbola et al., 2020). Therefore, the chelation of transition metal ions by peptides would delay the oxidation reaction, preventing the appearance of diseases such as neurodegenerative, cardiovascular, cancer, among others (Zhang et al., 2011).
Table 1 shows that the fraction that obtained the highest percentage of Cu2+ inhibition was F≤1 kDa (52.60 %), followed by F1-3 kDa, F3-5 kDa, and F5-10 kDa. These results are similar to those reported by Torres-Fuentes et al. (2011) for the fraction obtained from hydrolyzed chickpea protein.
On the other hand, low molecular weight peptides obtained from hydrolyzed chickpea protein with Cu2+ chelating capacity have been reported; these peptides present certain amino acids to which this capacity is attributed. Arcan and Yemenicioglu (2007) reported that some amino acids that present metal-chelating antioxidant activity are Lys, Arg, Asp, Glu, and His, likewise, Megías et al. (2007) obtained peptides that present His and Arg. Torres-Fuentes et al. (2011) reported peptide fractions with molecular weights between 0.1-1.2 kDa with 5-11 amino acid residues that are rich in Arg, Ser, and Lys. Zhang et al. (2011) obtained a peptide with a molecular weight 0.7 kDa and 5 amino acid residues (Asn, Arg, Tyr, His, and Glu).
In these studies, the antioxidant activity is attributed to the amino acid His due to the ability of the imidazole ring (which it possesses) to act as an H+ donor, likewise, Arg may show antioxidant activity due to its guanidino group (Arcan and Yemeniciog, 2007). Finally, Ghribi et al. (2015b) reported chickpea protein hydrolysates rich in amino acids such as Glu, Asp, and Arg, so that, as these amino acids are found in higher amounts with respect to others, and it is possible that the fractions obtained from hydrolyzed chickpea protein presented antioxidant activity since amino acids found in these fractions, are reported to have ABTS•+ and DPPH•+ radical scavenging capacity, iron-reducing power and metal chelating capacity. Other factors influence the antioxidant and biological activity of peptides, as these can be affected by the conditions used to obtain the protein concentrate, the degree of hydrolysis, the type of protease used, the structure and concentration of the peptide (Sarmadi and Ismail, 2010).
Conclusions
The combined use of the Alcalase-Flavourzyme enzy-mes generate extensive proteins hydrolysate that presented a higher antioxidant capacity than the protein concentrate. Of the four proteins hydrolysate fractions obtained by ultrafiltration, the first three presented anti-inflammatory capacity, inhibiting the production of NO, TNF-α and IL-1β. Furthermore, all fractions showed antioxidant capacity for the ABTS, DPPH, Fe3+ ion reducing capacity, and Cu2+ chelating capacity methods. This work demonstrates that it is possible to obtain peptides with anti-inflammatory and antioxidant activity from chickpea proteins hydrolysates. Nevertheless, it is important to consider that the fractions obtained from chickpea hydrolysate can contain other compounds that contribute or generate possible anti-inflammatory and anti-oxidant activity. Chickpea not only presents nutritional qualities such as its adequate protein content, but also biological activities, so it can be considered as a source of bioactive compounds that can be used as alternative therapies for certain diseases related to chronic inflammation. However, it is necessary that the peptide fractions obtained that presented the highest anti-inflammatory and antioxidant activity are sub-fractionated and characterized to identify the peptides responsible for these activities.











 nueva página del texto (beta)
nueva página del texto (beta)


