Introducción
Bacanora is a Mexican distilled beverage made from agave, similar to tequila and mezcal. The organoleptic characteristics of the beverage vary highly from producer to producer, due to the artisanal process used for production (Nuñez, 2001; Aguirre-Rivera et al., 2016). According to the Official Mexican Standards for bacanora, the drink must be made only from Agave angustifolia Haw and produced in the area of origin denomination for bacanora (AODB) (Alvarez-Ainza et al., 2009; D.O.F., 2005). The process is similar for mezcal and tequila production. First, the agave core is cooked in ovens with mezquite-wood, to hydrolyze the inulin, then milled to obtain the must called “saite” and then fermented by native yeasts. Finally, fermented agave must be distilled and standardized to the particular touch of each producer (Moreno, 1998; Núñez, 2001; Yanez, 2003; De la Cruz, 2003).
From the Mexican distilled beverages from agave (tequila, mezcal and bacanora), only tequila batches keep a constant sensorial quality, since it is produced in large facilities with high technology. Mezcal and bacanora, on the other hand, have large variations in sensory quality, since they are produced using artisanal process and the spontaneous fermentation driven by the combined action of various species of yeasts and bacteria. Furthermore, only a specific specie of agave is used for production of tequila, another kind for bacanora, and several different species for mezcal (D.O.F., 2004; D.O.F., 2005; Aguirre-Rivera et al., 2017). In the tequila industry, most companies use spontaneous fermentation, in many cases due to the natural-fermentation policy of the company. Different S. cerevisiae strains utilized for wine, beer or whisky fabrication, and even bakery yeast, are used as started in some tequila industries, although this yeast is able to ferment the tequila must, this is not the best suited to carry out this process (Gschaedler et al., 2004).
Starter cultures are required for keeping the typical quality of wine, however, pure cultures of yeasts from the same area are more effective (Querol et al., 2003). It is believed that these yeasts, called “selected local yeast”, are better because they are completely adapted to the climatic conditions, the raw material and they are partially responsible of the unique characteristics of the beverage (Mas et al., 2002; Querol et al., 2003). For example, in the study of Wondra and Berovic (2001), 29 native S. cerevisiae strains were evaluated during wine making, and found that only one of these strains produced an excellent wine, while 11 wines did not have good quality. The main desirable phenotypic characteristics to select a yeast culture, is resistance to adverse factors such as high concentrations of ethanol and low pH. In addition, other desirable characteristics are a short lag phase during fermentation, complete consumption of fermentable sugars and glycerol, and production of killer toxins. On the other hand, undesirable characteristics are production of H2S, volatile acids, and some of intermediate metabolites like acetaldehyde and pyruvate (Mas et al., 2002).
In addition, several studies have identified the importance of S. cerevisiae due to the role that it has on the modification of beverages. This could be depending on the inoculation process in the case of wine (Heart & Fleet, 1983), or on how this yeast can be found at different points of tequila production (Lachance, 1995).
In another study, Alvarez-Ainza et al. (2015) observed a high variability between the S. cerevisiae yeast isolated in every one of the municipalities during the natural fermentations process on the bacanora production. Moreover, this study showed how some non-Saccharomyces yeasts exhibited Saccharomyces-like characteristics, which might have an important participation on the fermentation process. In relation to this, Arellano et al. (2008) evaluated the kinetic parameters and the formation of volatile compounds from native S. cerevisiae and Kloeckera sp. strains, isolated from agave juice from tequila production, and observed that all S. cerevisiae strains as well as Kloeckera species had a similar behavior, however, between these species the production of aromatic compounds was different. Furthermore, Alvarez-Ainza et al. (2013) evaluated the main compounds of bacanora produced in the area with origin denomination, and found a great variability in the products; they recommend the use of a correct yeast culture as started for the fermentation process, so this variability could be corrected. In a Lopez-Alvarez et al. (2016) study, the fermentation process in the elaboration of tequila was evaluated, carried out by comparing an ethanol yield in a Kluyveromices marxianus native strain and in a bakery strain of S. cerevisiae, the results showed that the K. marxianus strain reached 96 % yield.
These studies support the idea that the Saccharomyces and non-Saccharomyces species play an important role in the fermentation process for tequila production, so they recommend the evaluation of mixed cultures. Therefore, the aim of this work was to select native yeast from a large group of S. cerevisiae and non-Saccharomyces strains isolated during the fermentation process of bacanora, and to evaluate the performance of the selected native yeast as started-mixed cultures for production of bacanora.
Materials and methods
Yeast Strain
Two hundred and five S. cerevisiae strains and 375 no-Saccharomyces yeast strains isolated from the fermentation tank of bacanora facilities, at different stages during the production of this beverage, were used (Collection of the laboratory of Microbiology in the Centro de Investigación en Alimentación y Desarrollo A. C., Hermosillo, Sonora, México). The yeasts were stored at -70° C in a YEPD medium (yeast extract 10 g/L, peptone 20 g/L and dextrose 20 g/L) with 30 % (v/v) glycerol.
Analysis of some phenotypical characteristics of yeast
Utilization of 1 % (w/v) glucose, fructose and inulin was assessed according to García-Galaz et al. (2004). Growth at 37 and 42° C was evaluated in PDA (Potato Dextrose Agar, Difco) plates acidified with tartaric acid 10 % (w/v) and incubated 48 h (D.O.F., 2004). Tolerance to ethanol was evaluated according to Lachance (1995), using 3, 5, 8, 10, 15 and 20 % (v/v) ethanol. Growth at different pH was evaluated on PDA plates, the initial pH was adjusted with tartaric acid in a pH range from 2.5 to 7.5, in 0.5 increments, and incubated at 30° C for 48 h. H2S production was evaluated on Bismuth Sulfite Agar (Difco) plates and incubating for 5 days at 30° C (Caridi et al., 2002). Killer factor was also evaluated using the killer-sensitive strain CECT 1018 and the Killer strain CECT 1891 (Caridi et al., 2002) as references. Yeasts were cultivated in YEPD broth 1.8 % (w/v), with fructose instead of glucose, absorbance at 600 nm was followed until stationary phase and the length of the lag phase was determined.
Evaluation of mixed cultures as starter for bacanora production
Juice from Agave angustifolia Haw. The juice from agave was obtained from the saite of a producer; the saite was mixed with water and all the juice was filtered and collected. The final sugar concentration was adjusted to 12° Brix (55±3 g/L reducing sugars) and stored at -10° C.
Pre-inoculum and inoculum. The selected S. cerevisiae and non-Saccharomyces (Torulaspora delbrucekii, Pichia membranefaciens and Kluyveromyces marxianus) strains were evaluated. Each strain was inoculated in diluted fermentation medium, from cultures of each yeast stored at -70° C in a YEPD medium, to obtain the pre-inoculum and the inoculum, the diluted agave juice (1:2 from an original of 12° Brix) was incubated for 12 h at 30° C in 500-mL Erlenmeyer flaks with 100 mL of agave juice, with low stirring (200 rpm). Two inoculums were used, one with three strains of S. cerevisiae (S. cerevisiae key 46, S. cerevisiae 591 and S. cerevisiae 1101) and another with three species of non-Saccharomyces (Torulaspora delbrucekii key 1227, Pichia membranefaciens key 807 and Kluyveromyces marxianus key 251).
Fermentation. Juice from Agave angustifolia Haw was sterilized in the fermentation tank (121° C, 15 min), the sugar concentration was adjusted at 12° Brix. The agave juice was supplemented with ammonium sulfate (1 g/L). Fermentations were carried in 3 L bioreactor (Applikon, The Netherlands) at 30° C and 200 rpm, using 2.5 L of the agave juice; air was not supplemented. Yeast was inoculated to an initial concentration of 20,000,000 cells/mL. Two fermentations were performed for each culture. The fermentation course was followed for 24 h and sampling was performed every hour during the first 12 h and each 2 h the remaining 12 h.
Analytical methods
Biomass concentration was estimated by total count using a Neubauer chamber or dry weight. Total reducing sugar was determined in previously hydrolyzed ferment yeast-free must by DNS method (Miller, 1959). Viability was estimated after dying cells with methylene blue (Díaz-Montaño et al., 2008; Segura-García et al., 2015). Ethanol concentration was determined using an enzymatic method measurement in an YSI biochemical analyzer 2700 (Yellow Spring Instrument). Karyotype technique was determined by pulsed clamp electrophoresis (PFGE) according to Alvarez-Ainza et al., (2015) with some modifications, using the zymoliase-20T enzyme at the first step of the process to degrade the cell wall (25 µg/mL, Seikaguru Corporation). S. cerevisiae YPH80 was used as molecular weight (Molecular Weight log Biolabs Inc.). The gel was stained with ethidium bromide and photograph with a GelDoc camera (BioRad).
Statistics analysis
The phenotypic characteristics analysis was conducted by BioNumerics software (Applied Maths 1998-2002), using UPGMA (Unweighted Pair Group Method with Arimetic Mean) and the DICE coefficient to obtain a dendrogram. The specific growth rate (µ), substrate consumed rate (rs), ethanol production rate (rp ), biomass and ethanol yields (Yx/s, Yp/s) as well as a maximal biomass production (Xmax) kinetic parameters were obtained by fitting a three-parameter logistic model for the fermentation with Saccharomyces, and the Gompertz model for the fermentation with non-Saccharomyces, based on the highest predictive fit obtained (R2), using the statistical package NCSS 2007(Kaysville, Utah, USA). The three-parameter logistic model states that: Y= A/ (1+B (EXP (-CT))), where Y is the number of organisms in a given time; EXP is the exponential function; A is the asymptote; B denotes the displacement in the X axis; C is the growth rate, and T is time. The Gompertz model states that: Y= A (EXP (-EXP (-B (T-C)))), where Y is the response variable; EXP the exponential function; A, B, and C are the parameters of equation, and T is time.
Results and discussion
Analysis of some phenotypical characteristics of yeast
All S. cerevisiae strains fermented glucose and fructose, but only 43 % (88 strains) fermented inulin. Of the non-Saccharomyces yeast, 8.2 % did not ferment glucose, 7.4 % were unable to ferment fructose and 57.3 % could use inulin. Of the S. cerevisiae strains, 94 % were able to grow at 37° C and 64 % at 42° C, and for the non-Saccharomyces yeast 97.4 % were able to grow at 37° C and 95.8 % at 42° C. All yeasts were able to grow and resist ethanol concentrations in the range of 3-15 %, and 95 % of S. cerevisiae yeast, as well as 95.7 % of the non-Saccharomyces strains, were able to resist 20 % ethanol. Only 8 S. cerevisiae and 170 non-Saccharomyces strains were unable to grow at pH 2.5. Regarding H2S production, 17 % of S. cerevisiae and 16.5 % of non-Saccharomyces yeast strains resulted positive. The killer phenotype was present only in three Saccharomyces yeasts, 14 were killer-sensible and 257 were killer-neutral.
The individual carbohydrate fermentation was performed in order to assess the ability of yeast strains to use these sugars individually. In this study, glucose, fructose and inulin, were assessed, which in theory are the agave juice sugars for the bacanora production (Aguirre-Rivera, et al., 2017); when cooked, agave “piñas” hydrolyze the carbohydrate reserve, those are the predominating with other fructans identified as new fructans or agavins (Mancilla-Margalli and Lopez, 2006). The bacanora process is done by a thermal treatment, however during the cooking process, all the fructans present in the “piñas” cannot be hydrolyzed, therefore, we also assessed whether these yeasts strains ferment inulin. In this study, it is noted that not all the non-Saccharomyces used glucose with a fermentative process, it is well know that this species uses most the oxidative metabolism (Heard and Fleet, 1985; Ciani et al., 2016a; Ciani et al., 2016b). The yeast tolerance or ability to grow at 37 and 42° C was important because in the region where the beverage is produced, temperatures of up to 42° are reached in summer, based on the reports for tequila and whisky fermentation (Diaz-Montaño et al., 2008; Walker and Hill, 2016). Optimum temperature for most yeast is 30 - 35° C, but this temperature may increase by 2 or 3 degrees during the process, and in summer, it has been observed that during tequila fermentation temperatures up to 40° C are reached (Diaz-Montaño et al., 2008). The yeast ability for ethanol tolerance was performed by the recommendations of Lachance (1995). In that work, most of the Saccharomyces strains resisted up to 11 % ethanol, and in the present work almost all yeasts resist 20 %. Moreover, in fermented musts of cane and agave juice between 4 and 6.5 % ethanol are reached, so the native strains involved in the process of developing bacanora have this great quality (Heard and Fleet, 1985). The pH of the “saite” is between 5 and 6, in some cases even lower (Fraizer and Westhoff, 1998), so in this study the survival of yeast at different pH was determined. Moreover, this study also evaluated the H2S production on each of the strains because it is non-desirable (Mas et al., 2002; Caridi et al., 2005). Some bacanora-producers state that the “saite” sulfur aroma (rotten eggs) during the fermentation generates economic losses, since the batch is discarded (personal communication with producers).
The killer phenotype was present only in three S. cerevisiae strains, 14 S. cerevisiae were killer-sensible and the rest of the S. cerevisiae and all the non- Saccharomyces (257 strains) were killer-neutral. Figure 1 shows the corresponding dendrogram analysis result of the phenotypic characteristics, and it shows the suitable characteristics recommended by Alvarez-Ainza et al. (2009) for bacanora production. Cluster 8 has the suitable characteristics recommended and it is constituted by 40 yeasts, 30 of them are S. cerevisiae, and 10 are non-Saccharomyces strains. Killer phenotype might represent an antagonist mechanism between yeasts during spontaneous fermentation process (Romano et al., 2003). The native populations might be a relatively high number of killer-resistant strains to the toxin and low presence of strains with killer-killer phenotype. Moreover, killer native populations in each area could put in interrogative the inoculation of a killer-sensitive strain selected to observe its best oenological characteristics. It is then recommended to select strains that show the killer-neutro o killer-killer phenotype, because it favors when are used as starter culture (Rodríguez et al., 1998).
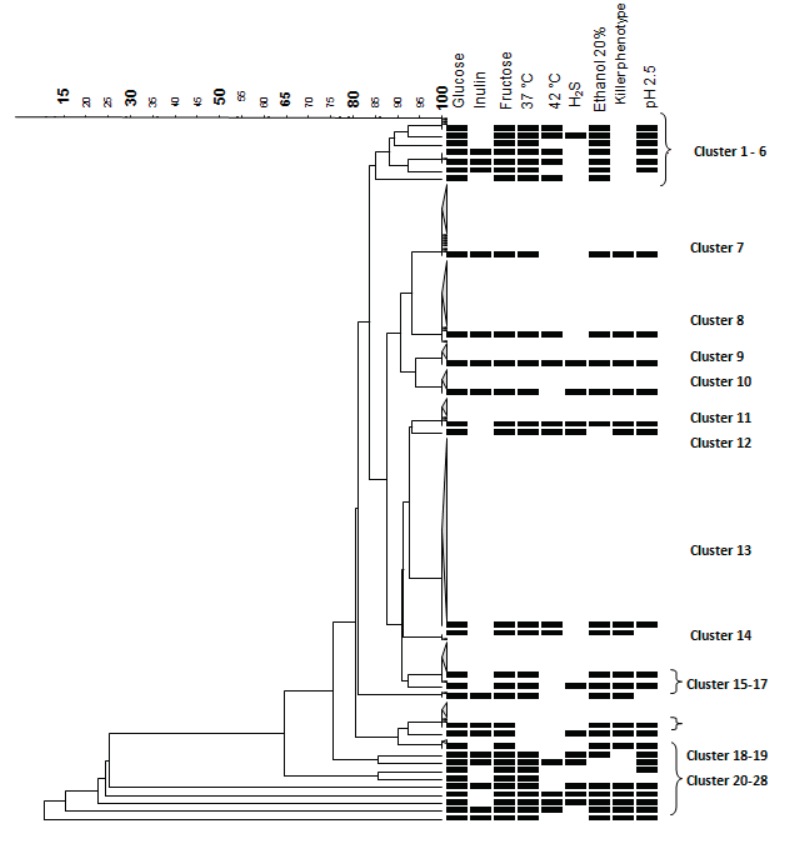
Figure 1 Phenotypic characteristics dendrogram of S. cerevisiae and
non-Saccharomyces yeasts, obtained by the
BioNumerics program using the coefficient Dice and UPGMA.
Figura 1. Dendrograma de las
características fenotípicas de levaduras S.
cerevisiae y no-Saccharomyces obtenido
por el programa BioNumerics, utilizando el coeficiente Dice y
UPGMA.
Figure 1 shows the results of the analysis performed by the BioNumerics program, where the coefficient Dice and UPGMA were used to obtain a dendrogram, where a group or cluster with the suitable characteristics recommended by Alvarez-Ainza et al. (2009) for the bacanora production, can be observed. This group is cluster 8, constituted by 40 yeasts, 30 of S. cerevisiae and 10 of non-Saccharomyces strains. After the analysis of the phenotypic characteristics, the growth curves analyses were done. These analyses showed that the latency phase was diverse in the different kinds of yeast, where the non-Saccharomyces show latencies phases of up to 5 hours and the S. cerevisiae strains show no more than 2.5 h. These results help to determinate the yeasts that are adapted to the medium with fructose, fermentable carbohydrate present in the agave juice (data not shown).
Later, three non-Saccharomyces yeasts with a latency phase under 2 h and different velocity of growth, were chosen for evaluation in mixed cultures. Likewise, 3 different species of non-Saccharomyces were chosen to be evaluated in mixed cultures: Kluyveromyces marxianus, Pichia membranefaciens and Toluraspora delbrueckii.
Evaluation of mixed cultures as starter for bacanora production
The time course of the Agave angustifolia Haw juice fermentation with Saccharomyces or non-Saccharomyces strains mixed cultures is shown on Figure 2, and the kinetic parameters on Table 1, where the P-value of the statistical analysis carried out with variance of kinetic parameters of the culture obtained using a logistic (S. cerevisiae culture) or Gompers model (non-Saccharomyces culture). The Saccharomyces mixed culture grew faster, and the maximal biomass had also greater numbers than the non-Saccharomyces, which yielded a maximal biomass of 8.27± 0.24 g/L, with reducing sugars depleted between 18-20 h. On the other hand, the mixed non-Saccharomyces strains culture yielded a maximum biomass of 3.87±1.14 g/L and reducing sugars were not depleted.
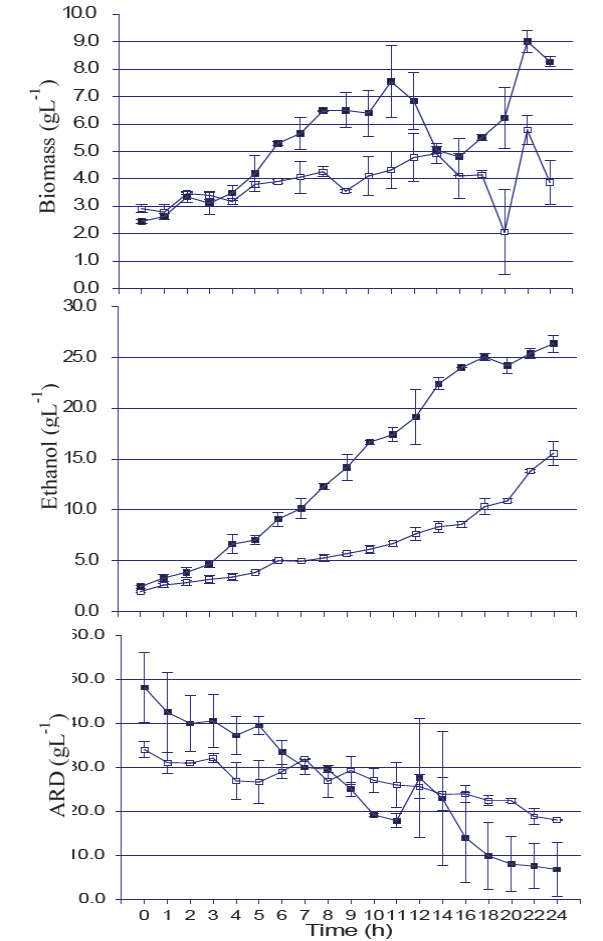
Figure 2 Fermentation kinetic profiles of cultures with S. cerevisiae (□) and
non-Saccharomyces (■). ARD: reduction sugar
concentration profile; Ethanol: ethanol concentration profile;
Biomass: biomass concentration profile (Mean ±standard
error).
Figura 2. Perfil cinético de la
fermentación de cultivos de S. cerevisiae (■) y no-
Saccharomyces (□). ARD: perfil de reducción de
la concentración de azúcares; Etanol: perfil de la concentración de
etanol; Biomasa: perfil de la concentración de biomasa. (Media
±error estándar).
Table 1 Kinetics parameters of the fermentation of agave juice inoculated with mixed cultures
of S. cerevisiae and
non-Saccharomyces strains.
Tabla
1. Parámetros cinéticos en la fermentación de jugos de
agave inoculados con cultivos mixtos de cepas de Saccharomyces
cerevisiae y no- Saccharomyces.
| Parameter | S. cerevisiae mixed culture | Non- Saccharomyces mixed culture | P value |
| µ (per h) | 0.29 ± 0.09 | 0.24 ± 0.06 | 0.6418 |
| rs (g/L/h) | 3.84 ± 0.12 | 1.03 ± 0.22 | 0.0008* |
| rp (g/L/h) | 1.76 ± 0.17 | 0.48 ± 0.03 | 0.0000* |
| Yx/s (g/g) | 0.07 ± 0.02 | 0.24 ± 0.03 | 0.0733 |
| Yp/s (g/g) | 0.45 ± 0.06 | 0.47 ± 0.03 | 0.8584 |
| Biomass (g/L) | 8.27 ± 0.24 | 3.86 ± 1.14 | 0.0331* |
| Total Alcohol (g/L) | 25.5 ± 1.19 | 15.56 ± 1.68 | 0.0179* |
| Fermentation time (h) | 18 | >24 |
µ: specific growth rate; rs, substrate consumed rate; rp, ethanol production rate; Yx/s, biomass yield; Yp/s, ethanol yield. Each value represents the average ± standard deviation of duplicate determinations of two fermentations. * Statistical difference: P < 0.05.
The Saccharomyces mixed culture (S. cerevisiae key 46, S. cerevisiae 591 and S. cerevisiae 1101) was more efficient than non-Saccharomyces (Torulaspora delbrucekii key 1227, Pichia membranefaciens key 807 and Kluyveromyces marxianus key 251) to produce ethanol. The first produced up to 25.51± 1.19 g/L with a volumetric productivity of 1.76±0.17 g/L/ h, while non-Saccharomyces strains produced 15.56 ± 1.68 g/L of ethanol with a volumetric productivity of 0.484±0.06 g/L/h. The kinetic parameter evaluated in the fermentations on this study, indicates the degree of adaptation of the microorganisms to the culture. The yeasts in mixed cultures were able to grow and achieve transformation of sugars into ethanol; it should be noted here that in the case of mixed cultures, there is also competition between the inoculated strains and the best adapted will be the predominant.
Similar results were reported by Arellano et al. (2008), they determined the kinetic parameters of wild yeast isolated from agave juice during tequila fermentation. In order to do this, they inoculated S. cerevisiae and Kloeckera sp isolates as monocultures in agave juice, and their results were in alcohol produced from 24 to 32 g/L being the S. cerevisiae specie the best alcohol producer. Similarly, Díaz-Montaño et al. (2008) study with Agave tequilana Weber juice, shows S. cerevisiae and Kloeckera with results between 21-43 g/L the etanol produced at the end.
Di Serio et al. (2003) evaluated bakery yeast on YEPD medium, and other medium made with molasses and different salts, reporting different results with respect to this study. Values reported by Di serio et al. (2003) on the maximum growth rate (1.26 ± 0.11 per h) and biomass yield per substrate consumption (0.723 ± 0.069 g/g), are higher than our results. This means that the YEPD medium and the molasses are more complete than the agave juice, or that the agave juice has inhibitors that affect the yeast, like some maillard compounds produced in the agave cooking, such as furfural. On the other hand, the results obtained are encouraging because the Bacanora producer obtained less than 20 g/L of ethanol in their fermentations, and in longer time with mixed S. cerevisiae cultures the production was 27.5 % higher.
The karyotypes of the yeast found at the end of the fermentation with mixed cultures (Figure 3), show that using S. cerevisiae, the strain with the key 1101 predominated at the end, but the other two also developed. In the non-Saccharomyces-mixed culture, Torulaspora delbrueckii predominated, and some Kluyveromyces marxianus strains were occasionally found, while Pichia membranefaciens was not found at the end, probably because this yeast is most sensible to alcohol or other products generated in the process. The yeast of Saccharomyces and non-Saccharomyces species are often found in alcoholic fermentations, for the production of wine, whisky and other agave beverages (Esteve-Zarzoso et al., 2001; Romano et al., 2003; Fiore et al., 2005; Diaz-Montaño, 2008; Fleet, 2008; Walker and Hill, 2016). The participation of the adequate Saccharomyces yeast has an important impact in ethanol production during fermentation, while the non-Saccharomyces yeasts in the final aroma; Torulaspora delbrueckii can be used to modulate sensory characteristics, such as acidity (Bely et al., 2008). Also, the use of S. cerevisiae is recommended to prevent native yeast growth on must, and the reduction of fermentation time (García et al., 2017). García et al. (2017) found that sequential cultures have produced more different wines providing organoleptic properties associated with the non-Saccharomyces strains used. Nolasco-Cancino et al. (2018) evaluated S. cerevisiae and non-Saccharomyces yeasts in single and mixed cultures in maguey juice (Agave angustifolia) used for mezcal production; the authors concluded that these cultures revealed their possible influence on volatile compound production, suggesting that the yeasts combination could contribute to improve the organoleptic characteristics of artisanal mezcal.

Figure 3 Karyotypes of yeasts found at the end of each culture probes fermentation. M:
molecular weight of S. cerevisiae YPH80; 1-3,
S. cerevisiae strains isolated at the end of
the fermentation; 4, K. marxianus control; 5, P.
membranefaciens control; 6, T. delbrueckii control; 7-10, strains
isolated at the end of the fermentation from the
no-Saccharomyces culture.
Figura
3. Cariotipos de las levaduras encontradas al final de la
fermentación de cada uno de los cultivos probados.
Conclusions
The native S. cerevisiae yeast isolated and selected from the Agave angustifolia Haw juice fermentation, can be used as starter mixed culture and the concentration of ethanol increases compared to the spontaneous fermentation in the production facility. Since Torulaspora delbrueckii ferments agave juice and predominated along the process in our study, it could be used together with S. cerevisiae to improve the fermentative process and enhance the sensory characteristics of bacanora but further studies are required.











 nueva página del texto (beta)
nueva página del texto (beta)


