INTRODUCTION
Mycobacterium tuberculosis (Mt), is the main agent that causes tuberculosis, is considered a public health problem, and the most lethal infeccious disease. Tuberculosis (TB), remains the world’s deadliest infectious illness around the world according to the Wolrd Health Organization (Syed et al., 2014; WHO, 2019) as a consequence of the inefficient clearance of pathogens, relapse of infection, as well as selection and propagation of drug-resistant Mycobacteria (Syed et al., 2014; Kulka et al., 2012). Urgent action is required to improve the coverage and quality of diagnosis, treatment, and care for people with drug-resistant TB. Treatment success for multidrug-resistant and rifampicin-resistant is currently low in many countries. This could be increased by improving access to new regimens that are more effective, less toxic, and easier to implement on eligible patients (WHO, 2019). Many other species of mycobacteria, including M. avium, M. fortuitum, M. marinum, and M. smegmatis, are well-documented. For instance, Mycobacterium smegmatis (Ms) has been reported as a causal venous-catheter bacteremia agent (Syed 2014 et al., Chakraborti and Kumar, 2019). The short generation time and low biosafety requirements of Ms, serves as an appropriate model to study Mycobacteria in general (Reyrat and Kahn, 2001) and is useful for anti-tuberculosis agents assays. Therefore, to obtain a Mycobacteria biofilm in the laboratory is an opportunity to characterize its synthesis and composition. In recent years, answers to these questions are emerging, but the entire picture is not as clear as desired (Chakraborti and Kumar, 2019). Some materials can work as a growth surface due to their physical and chemical characteristics. TiO2 and hydroxyapatite (HA), for example, have each been identified as a cell proliferation promoter (Baxter et al., 2009). Recently, Mt growth on Al2O3 and CaSiO3 porous supports has been reported (Hertog et al., 2010). Additionally, it is known that In2TiO5 have photocatalytic activity (Wen-Deng et al., 2007). UV-irradiated In2TiO5 acts via photocatalysis over human blood, increasing its oxygen concentration, a necessary element for Mycobacterium growth (Subrahmanyam et al., 2007). The aim of this work is to show the results on Ms growth over porous supports of a ceramic mixture, which were synthetized and characterized in our laboratories. On one hand, hydroxiapatite (HA) was considered for the preparation of these supports, due to its good promotion of bacterial growth and the reported good cell adhesion to HA’s porous surface (Annaz et al., 2004). On the other hand, indium titanate is a ternary oxide, its applications are primarily as a filter (bypass), a microcapacitor, a refractory ceramic, and as a photocatalyzer. Our group studied the thermoluminescence properties of indium titanate europium activated, as a possible radiation dosimeter (Muñoz et. al. 2016). Looking for new applications for this ceramic, we considered it as a supports component due to its photocatalytic properties. To our knowledge, there are no previous reports on biofilm growth of Mycobacterium on any of the aforementioned ceramic materials (HA and In2TiO5).
MATERIALS AND METHODS
Synthesis of Supports
The highest purity reagents commercially available, In2O3 (99.9% Rare Metallic Co., Ltd.) and TiO2 (99.9% Rare Metallic Co., Ltd.), were mixed in 1:1 molar ratio, and grounded in an agate mortar. The mixture was molded into pellets and annealed in air at 1250 °C in a ThermolyneTM 1300 furnace for 48 h. Then, In2TiO5 was obtained through conventional solid state reaction similar to previously-reported methods (Muñoz et al., 2016; Senegas and Galy, 1975). Comercially-available hydroxyapatite (HA reagent grade 36.2 % Ca Aldrich) was used to form a HA+In2TiO5 mixture at a 1:1 mass proportion. A total mixture mass of 50 mg of HA+In2TiO5 were prepared in a cylindrical shape in a Carver® model C hydraulic press using 0.5 ton for 10 s. Next, supports were synthetized at 1200 °C for 48 h, resulting in rigid supports.
Characterization
X-ray powder diffraction (XRD) patterns were recorded at room temperature on an X-ray powder diffractometer Rigaku® Geigerflex D/MaxB with a Cu anode and a graphite monochromator operated at 40 kV, 20 mA, a scan speed of 2°·min-1 in 2 tetha on the interval of 10° to 70°. Scanning electron microscopy (SEM) images and energy dispersive spectra were acquired using a JEOL JSM-5410LV scanning electron microscope, equipped with an Oxford X-ray Energy Dispersive Spectroscopy (EDS) analyzer operating at 20 kV. The oxygen concentration was analyzed in a Fisher ScientificTM Oxygen Meter using aerated Middlebrook (MDB) 7H9 medium as a control.
Ms Growth on Ceramic Mixture Supports with MDB 7H9/OADC
The ceramic supports, were placed in each well of a sterile flat-bottom 96-well plate with 100 µL of Middlebrook 7H9 broth supplemented with OADC (oleic acid, albumin, dextrose, and catalase) (7H9-S) as culture media, and 100 µL of bacterial inoculum adjusted using the turbidity McFarland standard 1 (3×108 CFU/mL), further diluted at a 1:50 ratio in 7H9-S broth for the test. The plate was covered, sealed in a plastic bag, and incubated at 37 °C under normal atmosphere. After 2 days of incubation, the supports were fixed with formaldehyde at 10% for 2 hours and then were analyzed by SEM.
Resazurin Microtiter Assay Plate (REMA)
The resazurin reduction and the M. smegmatis growth were determined as previously described (Khalifa et al., 2013) with modifications. The final drug concentration tested was 64 μg/mL for gentamicin. After 2 days of incubation, 30 μL of resazurin solution was added to each well, and the plate was reincubated overnight. A change from blue to pink indicates resazurin reduction and, therefore, bacterial growth. The minimal inhibitory concentration (MIC) was defined as the lowest concentration of the drug that prevented this change in color. The assays were performed using the supports with solar light exposure during 30 min and 60 min, prior to being inoculated with M. smegmatis. The sunlight exposition was carried out in Hermosillo Sonora, México; during spring, at an ambient temperature of around 38 °C and UV index ranging between 8 and 10.
RESULTS AND DISCUSSION
Supports Characterization
The ceramic supports were prepared with HA and In2TiO5 in 1:1 mass proportion. The supports are white color and extremely fragile before synthesis (Figure 1), but after they become more rigid and their color turns slightly gray. Its dimensions are reduced in area and height to 4 mm in diameter and 2.5 mm in height. In Figure 2, the powder XRD of a sample of cylindrical support synthetized at 1200 °C for 48 h is shown. According to X-rays patterns, the supports were composed by Ca3(PO4)2 [01-070-2075, Rombohedric]; In2TiO5 [01-082-0326, Orthorombic]; CaTiO3 [01-076-2400, Orthorombic] and In2O3 [01-074-1990, Cubic C-Type].

Figure 1 Ceramics supports before and after the 48 h-synthesis at 1200 °C.
Figura 1. Soportes cerámicos antes y después de la síntesis a 1200 °C durante 48 h.
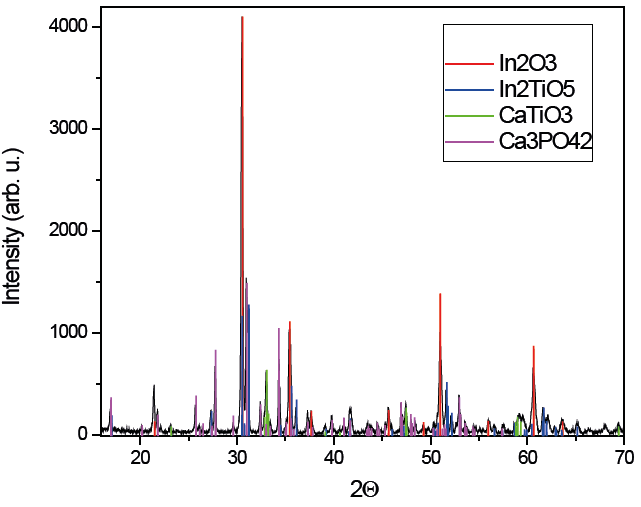
Figure 2 X-ray diffraction of a ceramic support synthetized at 1200 °C for 48 h, compared with Ca3(PO4)2 [ICCD card 01-070-2065], In2TiO5 [ICCD card 01-082-0326], CaTiO3 [ICCD card 01-086-1393], and In2O3 [ICCD card 01-076-0152].
Figura 2. Difracción de rayos X de un soporte sintetizado a 1200°C durante 48 h comparado con las tarjetas de la base de datos de ICCD de Ca3(PO4)2 [ICCD 01-070-2065], In2TiO5 [ICCD 01-082-0326], CaTiO3 [ICCD 01-086-1393], y In2O3 [ICCD 01-076-0152].
To elucidate these results, we propose that HA (25 mg) and In2TiO5 (25 mg) undergo a reaction during its annealing at 1200 °C/48 h:
Reaction 1 Ca5O13P3 + In2TiO5 → In2TiO5 + In2O3 + Ca3(PO4)2 + CaTiO3
The stoichiometry of the reaction: 4.99×10-5 mol of HA and 6.99×10-5 mol of In2TiO5 reveals HA as the limiting reagent, which is consistent with the proposed reaction. The phases shown by XRD were confirmed by SEM and EDS. Figure 3 presents a microphotograph of the sample obtained by backscattered electrons. We can see syntered bodies with at least four different contrasts corresponding to four different phases. Based on the EDS data, these correspond to phases Ca3(PO4)2, In2TiO5, In2O3 and CaTiO3.
Ms Growth on Ceramic Mixture Supports
Growth in communities appears to be a preferred survival strategy of microbes and is achieved through genetic components that regulate surface attachment, intercellular communications, and synthesis of extracellular polymeric substances (O’Toole and Kolter 1998; Henke and Bassler 2004; Syed et al., 2014). There are some reports regarding the strong cellular aggregation and rapid formation of the Ms biofilm (Shi Fu et al., 2011; Bonkat et al., 2012; Ojha et al., 2005). Scanning electron microscopy (SEM) revealed that Ms exhibits strong cellular aggregation and dense material (biofilm) formation (Syed et al., 2014; Tingyu et al., 2011). The micrographs obtained here from supports after the incubation and M. smegmatis growth, revealed biofilm formation attached to the surface of the ceramic support (Figures 4a and 4b). These micrographs were compared with micrographs of ceramic supports without the addition of bacterial inoculums, in which a porous surface was observed. The porosity has been reported as a promotor of bacterial growth and cell adhesion to some surfaces (Annaz et al., 2004; Bagambisa and Joos, 1990; Salem, 2002).
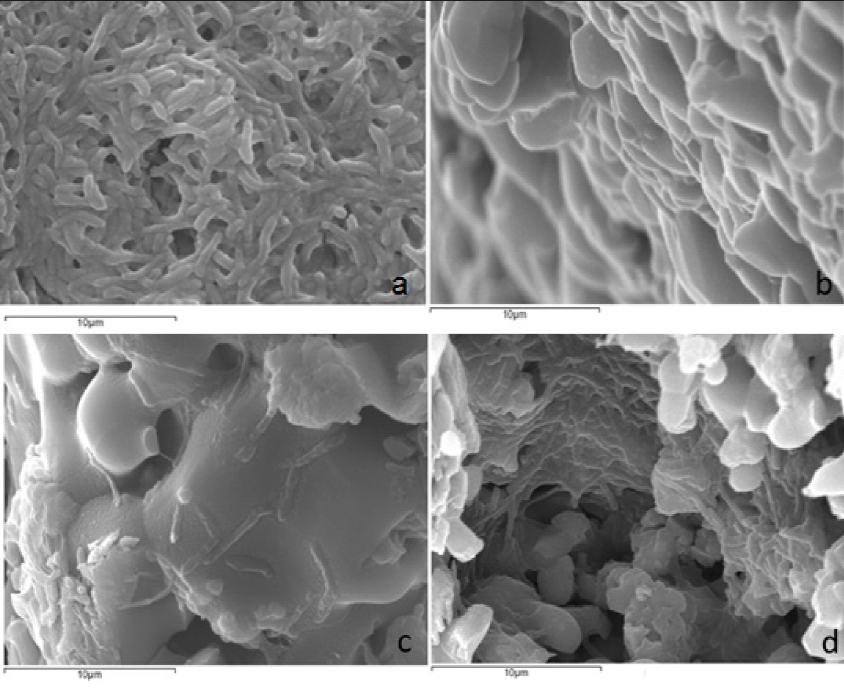
Figure 4 Micrographs of ceramic support X5000: general view (a); M. smegmatis biofilm over a support (b); M. smegmatis bacillus embedded into the biofilm (c); M. smegmatis bacterial conglomerates and biofilm formation over ceramic mixture support (d). Samples were incubated at 37 °C/48 h.
Figura 4. Micrografías de soportes cerámicos X5000: vista general (a); biopelícula de M. smegmatis sobre un soporte (b); bacilos de M. smegmatis embebidos dentro de la biopelícula (c); conglomerado de bacterias y formación de biopelícula de M. smegmatis sobre el soporte de mezcla cerámica (d). Las muestras fueron incubadas a 37 °C/48 h.
Figure 4c shows the biofilm where bacteria are embedded in a matrix of extracellular polymeric compounds. The bacterial conglomerates were observed inside the pores and spread throughout the surface of the support (Figure 4d). Ms is known to form well-organized colonies and microcolonies which have been described by Danese et al. as a type of biofilm (qtd. in Chakraborti and Kumar, 2019). These colonies are composed of microbial cells encapsulated by a large amount of exopolysaccharides (Chakraborti and Kumar, 2019).
Resazurin Microtiter Assay Plate (REMA)
Isolates of M. smegmatis were assessed using REMA on supports for resistance to gentamicin in Middlebrook 7H9 broth by cultivation on plates containing serial dilutions of gentamicin.
The MICs obtained were compared to REMA assays with and without supports of ceramic mixture and assays with Ca3(PO4)2 supports (Table 1). An increment on the MIC of gentamicin that inhibits M. smegmatis is related with a favorable growth impact, attributed to the ceramic mixture supports. The MIC of assays with supports was higher by a factor of 4 compared to the MIC of assays without supports, and also twice as high as the MIC with Ca3(PO4)2 supports.
Table 1 Gentamicin minimum inhibitory concentration (MIC) against M. smegmatis using supports exposed to sun light (SL, min) compared to controls without SL exposition.
Tabla 1. Concentración minima inhibitoria (MIC) de gentamicina contra M. smegmatis utilizando soportes expuestos a la luz solar (SL, min) comparado con los controles sin exposición a SL.
| Support | MIC (µg/mL) |
|---|---|
| Without support | 0.5 |
| Ca3(PO4)2 | 1.0 |
| Ceramic mixture | 2.0 |
| Ceramic mixture + SL 30 | 4.0 |
| Ceramic mixture + SL 60 | 4.0 |
Photocatalysis Induced by Sunlight
In order to evaluate the photocatalytic effect induced by the supports on M. smegmatis growth, REMA assays were performed with 30 and 60 min of exposure to sunlight prior to the bacterial inoculum. The MICs of assays employing supports with different sunlight exposure times, were the same, but these were higher by a factor of 8 compared to the MIC obtained on assays without supports (Table 1). An increase of oxygen concentration has been reported on different systems where titanium-containing materials, such as In2TiO5, have had a photocatalytic effect (Subrahmanyam et al., 2014). Mycobacteria are strictly aerobic organisms and the oxygen generated by the material might have been used by the bacteria and favored their growth and the formation of biofilm. How does the mycobacterial biofilms form and what induces their formation have remained important questions in the field. In the last two decades, understanding the mycobacterial biofilms has evolved into a niche area for research (Chakraborti and Kumar, 2019).
The dissolved oxygen in MDB 7H9 was determined using Winkler bottles and ceramic supports, after the sunlight exposure. The dissolved oxygen concentration increased by 35% on the ceramic supports-containing culture medium after being exposed to sunlight for 30 min. The maximum concentration of dissolved oxygen was 20% higher than the support-less control and it is attributed to the photocatalytic effect of In2TiO5, a component of the supports immersed in the culture medium (Figure 5).
CONCLUSION
The proposed ceramic supports based on HA+ In2TiO5 showed a considerable effect on M. smegmatis growth, being 8 times higher when the material was exposed to sunlight. This is attributed to the increase of oxygen on the culture medium, via photocatalysis promoted by In2TiO5 present in the supports. The use of this ceramic support showed the Ms growth 24 h earlier with respect to the suportless control, which is an important time save on the diagnosis of Mycobacterium. An additional advantage of those supports is that bacteria can be manipulated after culture and can be processed by imaging or microscopy methods. The material could be incorporated into an agar plate and be used with other microbial pathogens to study the effect of drugs or bacteriostatic agents. The ceramic supports could be used as a microarray platform for molecular assays, offering the prospect of coupling bacterial culture to molecular tests.











 nueva página del texto (beta)
nueva página del texto (beta)




