Introduction
The control of plant diseases is the most challenging aspect of crop production. In recent years, the resistance to pesticides available in the market has increased and became a serious problem (Chattopadhyay et al., 2017; Goffeau, 2008). Among the microorganisms related to plant diseases, fungi cause a higher percentage in agricultural systems. Fusarium wilt is a fatal vascular wilt syndrome in plants belonging to the Solanaceae family (Omar & Ahmed, 2014).
The fungus F. oxysporum comprises more than 120 known strains or special forms, each of which is specific to a single host plant in which it causes disease. Fusarium oxysporum f. sp. Lycopersici cause wilt in tomato. Fusarium wilt is a destructive tomato disease in several countries of the world, and is of great concern to producers due to its large loss of production, fungi prolonged survival in the soil and generation of resistant strains. The disease can be reduced to some extent with the use of resistant cultivars and chemicals. However, the evolution to new pathogenic strains is a continuous problem, and the use of chemical products is expensive and not always effective (Amini & Sidovich, 2010; Malandrakis et al., 2018). Therefore, the control mechanism for this type of diseases, with no harmful effects for crops nor to the environment, has been a major concern for researchers working in the field of disease management.
Therefore, it is of immediate necessity to look for alternative disease control measures that are safe for the environment. In recent years, the use of nanomaterials is expanding and has been considered as an alternative solution to control plant pathogens. Some metallic nanoparticles have been studied and tested for their antifungal properties (Al-Huqail et al., 2018; Janaki et al., 2015; Medda et al., 2015; Shaikh et al., 2019). Several studies have demonstrated the effectiveness of AgNP against phytopathogenic microorganisms. Jo et al. (2009) demonstrated that AgNP were effective in reducing the growth of Bipolaris sorokiniana and Magnaporthe grisea fungi in vitro and reducing the severity of the disease in plants by applying the nanomaterial 3 h after the inoculation with spores, but their efficacy decreased significantly when applied after 24 h of inoculation. Similarly that AgNP were effective against various phytopathogens such as Alternaria alternata, Botrytis cinerea, F. oxysporum and Pythium spinosum (Kim et al., 2012). Mishra et al. (2017) showed the antifungal activity of AgNPs against foliar and soil-borne phytopathogens. The inhibitory impact of different silver concentrations (2, 4, 10 μg/mL) on conidial germination was recorded under in vitro conditions. In another study, Karimi and Sadeghi (2019) isolated 3 different phytopathogens (Alternaria alternata, Alternaria citri and Penicillium digitatum) from citrus samples. In vitro assay was carry out on PDA media treated with 50, 100 and 150 ppm of silver nanoparticles and two conventional fungicides. Results revealed that Ag-NPs showed stronger antifungal activity against the isolated fungi that conventional fungicides.
However, these authors did not evaluate the nanoparticles in vivo. In the literature there is a lot of information about the antifungal effect of the AgNP, however, there is a lack of information about the effect on the plant and the development of the disease at field level, as well as the combination with other materials or polymers that make the nanostructure more stable and improve its properties. Therefore, the antifungal effects of Ag-Cs NPs on Fusarium wilt in tomato seedlings were investigated in the present study under in vitro and greenhouse conditions.
Materials and methods
Materials
All the reagents were of analytical grade and used without any further purification. Silver nitrate (AgNO3), sodium borohydride and Chitosan of LMW were purchased from Sigma-Aldrich with a ≥99.5% purity. The wild fungi Fusarium oxysporum was isolated from infected plants in the Costa de Hermosillo, the strain was subcultured in potato dextrose agar (ThermoFisher scientific) for morphological identification.
Synthesis and characterization of silver nanoparticles
The silver nanoparticles coated with chitosan (Ag-CsNPs) were synthesized according to the methodology proposed by Bin Ahmad et al. (2011). The chitosan solution was prepared by solubilizing 1.0 g of chitosan in 50 mL of acetic acid. Immediately afterwards, AgNO3 (50 mL, 0.01 M) was added to the chitosan suspension under constant stirring for 2 h. Subsequently, 20 mL of NaBH4 (0.04 M) were added to the AgNO3-chitosan suspension, a color change from pale yellow to brown was immediately observed indicating the formation of the nanoparticles. The nanoparticles were characterized by evaluating the surface charge by means of a zetaseizer (Malvern Instruments) and the morphology and size were observed by transmission electron microscopy.
In vitro evaluation of the antifungal capacity of Ag-Cs NPs agaisnt Fusarium oxysporum
The antifungal effect of Ag-Cs NPs against the fungus F. oxysporum was evaluated. For this, the nanoparticles were dissolved at different concentrations (1000, 1500 and 2000 ppm) in sterile PDA medium at 45 °C and then, the medium was added to Petri dishes to solidification. The medium without addition of the nanoparticles was considered as the control treatment. Subsequently, F. oxysporum was inoculated from a culture of 7 days old in the center of the Petri dish. They were incubated at 25 °C for 7 days. Each treatment was performed in triplicate. The diameter of mycelial growth in vitro was determined, which was measured at 48, 96 and 168 h after the start of the experiment using a vernier and the results were expressed in cm.
Evaluation of Ag-Cs NPs in development of tomato seed-lings
To determine if the of Ag-Cs NPs exerted any toxic effect, different concentrations of the treatment on tomato plants were evaluated. Tomato seedlings used in this experiment were 20 days old after germinated. On the substrate without or with 3% earthworm humus, 50 mL of the nanoparticles were applied at the concentrations of 1000, 1500 and 2000 ppm every 7 days for 30 days. The height and diameter of the plants were measured, as well as dry and fresh weight. Each treatment was carried out in triplicate.
Effect of NPs of Ag-Cs on the development of vascular wilt in tomato seedlings inoculated with Fusarium oxysporum
The bioassay was developed by preparing a spore suspension of the fungus at a concentration of 1 x 107 spores per mL with a 30% glucose solution. Subsequently, a small wound was made at the neck of the root to facilitate the infection, and the seedlings of 20 days after germination, were immersed in the spores suspension for a period of 4 h. Seedlings were placed in Petri dishes filled with Peatmost® and incubated in a growth chamber for 14 days with 90% relative humidity at 25 °C. The nanoparticles were applied on the soil at a concentration of 2000 ppm five days after the inoculation. In the experiment, there was control of the disease, treatment control without disease and treatment with nanoparticles. Each treatment was performed with 10 repetitions. After 14 days the severity of the lesions caused by the disease, the height and diameter of the plants and the activity of the catalase enzyme were evaluated.
Statistical analysis
The experiments were carried out under a completely randomized design. To determine the effect of the treatment on the response variables, an analysis of variance of one or two routes was carried out, depending on the case, with a confidence index of 95%. The difference between the means of the treatments was compared using the Tukey-Kramer test. Differences at P <0.05 were considered significant. All this using the NCSS 2007 statistical package.
Results and discussion
Synthesis and Characterization of Nanoparticles
Nanotechnology has generated great interest in the development of agricultural products, since it represents an excellent opportunity to reduce the use of synthetic agrochemicals, with the possibility of reducing the environmental impact, and enhance the uptake of nutrients from the soil. Figure 1 shows the TEM micrographs of the NPs stabilized with chitosan and the size distribution of elaborated nanoparticles, since the morphology and size are extremely important to predict if the plant will be able to capture and biodistribute the NPs. The TEM images of the sample show spherical morphologies with less than 2% polydispersity, with a mean diameter of 33.26 ± 6.15 nm (Figure 1b). The functionalization and stabilization with chitosan can be clearly seen in Figure 1a with the contrast difference.

Figure 1 TEM micrographs of the Ag-Cs NPs. a) approach 250,000 x of the nanoparticles, b) size
distribution of AgNPs-CS.
Figura 1. Micrografías TEM de las AgNPs-Cs. a)
acercamiento 250,000 x de las nanopartículas, b) distribución de
tamaño de AgNPs-CS.
A number of variables, such as particle size, AgNP surface charge and the physicochemical features of the soil, largely govern the chemistry, fate and transport of the AgNPs in the soil system and, therefore, its bioavailability and its sub-sequent bioaccumulation in plant tissue (Grün et al., 2019). A retention of AgNP has been evidenced in soil suspensions that correlated mainly with the clay content of the soil, and the authors advocated the role of the heterocoagulation of AgNP with natural colloids to explain this correlation (Grün et al., 2019; Tolaymat et al., 2010). Torrent et al. (2019) evidenced that cation exchange capacity and electrical conductivity are the main parameters controlling the adsorption processes in soil of AgNP with different sizes (45, 75, 200 and 200 nm) and coated with polymers of different charge (citrate, polyvi-nylpyrrolidone and polyethyleneglycol (PEG))
It has been reported that several stabilizing agents modify the surface properties of AgNP where their mobility can be altered by the electrostatic interaction of different types of soil. For example, a positively charged soil can prevent long-distance mobility of citrated AgNP negatively charged. Conversely, a negatively charged soil can make the AgNP more mobile in such soils. In this study we used Cs as a stabilizing agent and its surface charge was evaluated resulting in a potential ζ of around 50 (+), as a result of the polymer chains amino groups ionization. This promotes the absorption by clays for later incorporation by the plant, by cation exchange (Thio et al., 2011).
Antifungal Activity of Nanoparticles
Figure 2 shows the in vitro antifungal capacity of Ag-Cs NPs against the phytopathogenic fungus F. oxysporum. The results show a larger diameter of the fungus colony at longer incubation times, which was expected since the conditions are ideal for the fungus growth. The highest fungal growth occurred in the control treatment with a final diameter of 50 mm. On the other hand, the nanostructures were significantly effective to inhibit mycelial growth, comparing it with the growth of the untreated fungus (control) (p <0.05). Among the treatments, the nanoparticles at the 1000 ppm concentration managed to reduce the growth of the fungus by 64%, while the concentrations of 1500 and 2000 ppm were more effective (p <0.05) since they managed to reduce fungal growth, 70% and 74% respectively, without statistical differences between them.
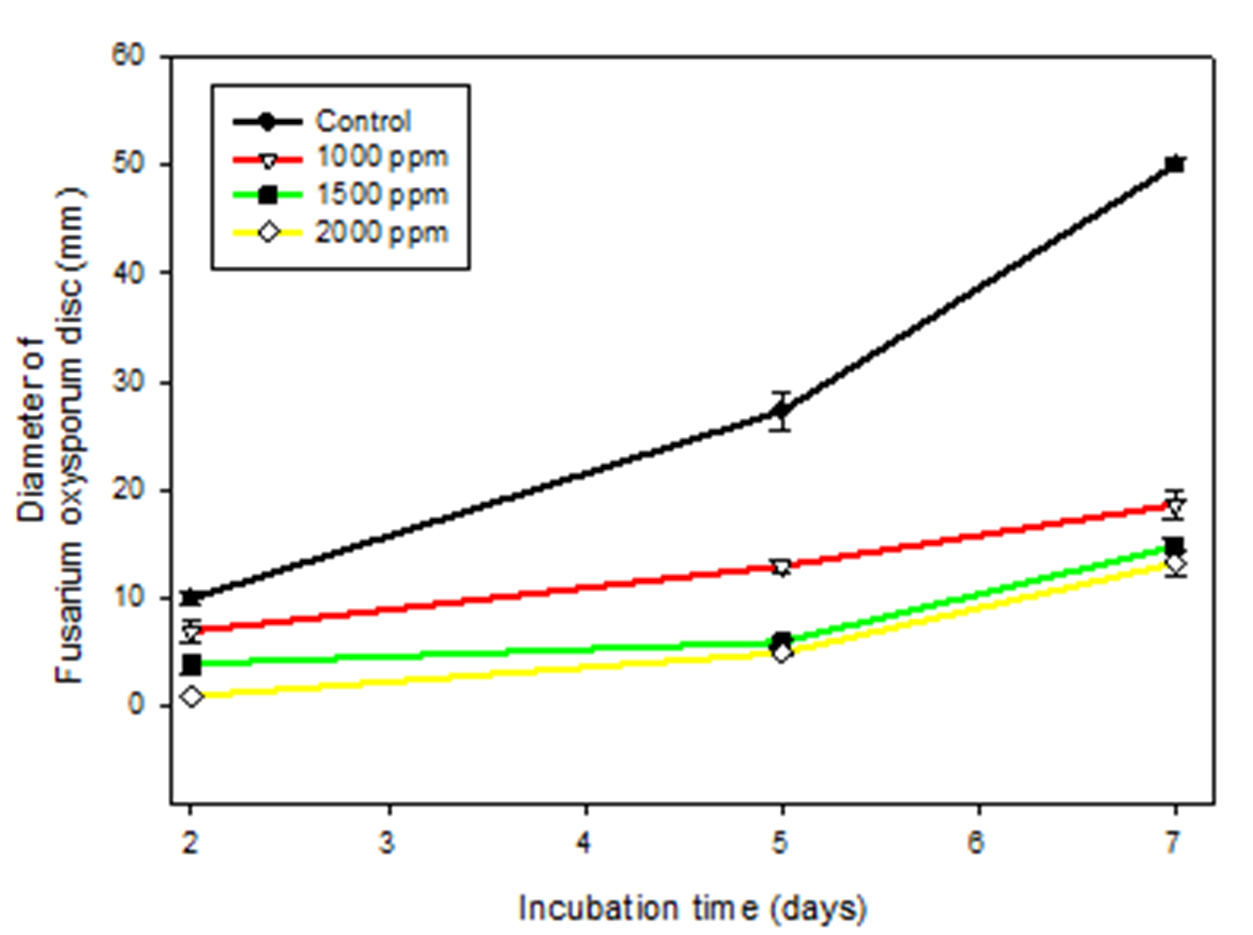
Figure 2 Antifungal capacity in vitro of silver nanoparticles coated with chitosan against
Fusarium oxysporum in an incubation period of 7 days at 25 ° C (p˃0.05).
Figura 2. Capacidad antifúngica in vitro de
nanopartículas de plata recubiertas con quitosano contra Fusarium
oxysporum en un periodo de incubación de 7 días a 25 °C.
For many decades, silver (Ag +) has been studied in its use in disinfection processes against several pathogenic microorganisms, since it has environmentally friendly characteristics and a powerful antimicrobial capacity. Kim et al. (2012) evaluated the use of AgNP at concentrations of 10, 25, 50 and 100 ppm against 18 phytopathogenic fungi on potato dextrose agar. The authors found a dosedependent antifungal effect, observing 100% growth inhibition of the fungi Alternaria brassicola, Cylindrocarpo destrutans, Fusarium sp, Pythium aphanidermatum and Pythium spinosum at the highest concentration (Kim et al., 2012).
In another study, the antifungal activity of Ag, copper and the mixture of these NPs against two phytopathogenic fungi, A. alternata and Botrytis cinerea was investigated (Ouda, 2014). In this study the metallic nanoparticles were applied at different concentrations, finding that at 15 ppm produced the maximum inhibition of the fungal hyphae growth. At this concentration the AgNP inhibited the growth by 59.3 and 52% of A. alternata and B. cinerea, respectively, being more effective than the copper nanoparticles and their mixture. It has been proposed that the antimicrobial effect of AgNP is different according to the microbial species.
The AgNP mode of antifungal action can be through morphological, structural and physiological changes, altering the cell membrane, affecting the fungus hyphae and conidia, producing reactive oxygen species (ROS) that cause damage to proteins, lipids and nucleic acids (Ouda, 2014). Dasgupta and Ramalingam (2016) showed that the main mechanism of nano-silver formulations is ROS generation and increase in membrane permeabilization. In addition, they can have a detrimental effect on sugar, proteins, nacetyl glucosamine and lipids of the cellular filtrate and cell wall components of phytopathogens. They have also been associated with the union and penetration in the cell membrane to kill spores, although this mechanism is not completely known (Pietrzak et al., 2015). Kumari et al. (2019) demonstrated that after membrane disintegrity, the cells lead to death by loss of membrane structure and osmotic balance in AgNP treated fungus; additionally, they observed collapse of redox homeostasis, antioxidant machinery, cellular virulence and damage of cell wall and membrane by disrupting osmotic balance as major mechanisms.
Evaluation of Ag-Cs NPs in development of tomato seed-lings
In order to ensure that the nanoparticles did not have any harmful effect on the tomato plants, we evaluated their effect, at different concentrations, on the size of the plant in height and diameter of the stem and dry weight of leaves, stem and root. In addition, this was done in the absence or presence of earthworm humus in order to improve the cation exchange of the substrate (Berilli et al., 2018).
Figure 3 shows the results of tomato seedling height after being exposed to Ag-Cs NPs with and without 3% humus. Three different doses of the treatment were compared (1000, 1500, and 2000 ppm) and it can be observed that the height of the control and treated seedlings with the doses of 1000 and 1500 ppm with and without humus and the 2000 ppm without humus did not show significant difference between them (p <0.05). On the other hand, the seedlings to which the highest concentration with humus was applied showed greater development after 30 days of the application, presenting a significant difference with the control and the rest of the treatments with and without humus (p <0.05). These results indicate that silver nanostructures have no toxic effect on plants and on the contrary it seems to benefit their development.

Figure 3 Height of tomato seedlings exposed to different concentrations of silver nanoparticles
coated with chitosan at 30 days after application.
Figura 3. Altura de plántulas de tomate expuestas a
diferentes concentraciones de nanopartículas de plata recubiertas de
quitosano a los 30 días después de la aplicación.
Figure 4 shows the results of dry weight and fresh weight of root, stem and leaf of tomato seedlings after 30 days of the first application of Ag-Cs NPs in the presence and absence of earthworm humus. It can be observed that the control treatment had the lowest dry and fresh weight while there is a significant difference due to the effect of the nanoparticles; although there is no clear trend in the concentrations of the treatments. Comparing the effect of earthworm humus, the presence of humus significantly favors the increase in tomato seedlings biomass with the three nanoparticles concentrations. This can be attributed to the fact that earthworm humus allows a greater occurrence of cation exchange reactions and this improves the absorption of silver particles in the soil (Anjum et al., 2013; De Farias et al., 2018).

Figure 4 A) Dry weight and B) Fresh weight of tomato seedlings (stem, root and leaf) exposed to
different concentrations of silver nanoparticles coated with
chitosan at 30 days after application. Treatment 1 is with earthworm
hummus and Treatment 2 is without earthworm hummus. p <0.05
indicates significant differences between all treatments.
Figura 4. A) Peso seco y B) Peso fresco de plántulas de
tomate (tallo, raíz y hoja) expuestas a diferentes concentraciones
de nanopartículas de plata recubiertas de quitosano a los 30 días
después de la aplicación. p <0.05 indica diferencias
significativas entre todos los tratamientos.
The results of our study showed that the humus increases the height of the plants at high silver concentrations, since it plays a role in the absorption of silver ions by the root, due to the improvement in the capacity of cation exchange in the soil, which allows a greater mobility of Ag+ ions and retention of these to be taken by the rhizosphere.
Several studies have evaluated the effect of AgNP application on the vegetative development in different crops. Raliya et al. (2015) applied zinc oxide and titanium nanoparticles on tomato crops, finding significant differences in vegetative development with a 25% increase in the best treatment. Likewise, AgNP-Cs (0.1% w / v) showed a growth promoting effect on the germination of chickpea seed, seedling length, fresh weight and dry weight (Anusuya & Banu, 2016). In addition, the treatment increased the chlorophyll content and the activity of the α, β-amylase enzymes, ascorbate peroxidase, peroxidase and catalase. The authors report that the possible reason for the growth rate increase, is a greater absorption of inorganic nutrients that accelerated the decomposition of organic substances during the photosynthetic process, which increased the rate of photosynthesis. In addition, the key to increase the germination rate of the seed is the penetration of nanomaterials in the seed (Anusuya & Banu, 2016).
However, some studies report adverse effects with the application of AgNP. Song et al. (2013) found a decrease in the biomass of tomato seedlings at the lowest concentration evaluated. In another study, Lupinus termis L. seedlings were exposed to 0.5 mg/L of AgNP for ten days, with significant reductions observed in growth parameters such as sprouting, root lengthening and fresh weight (Al-Huqail et al., 2018). In addition, the treatment caused metabolic disorders such as reduction of total chlorophyll, sugars and proteins content. This can be attributed to the characteristics of the plants, the release of toxic ions, as well as the size and shape of the particles that stimulate stress. However, at lower concentrations these effects were not observed.
In summary, the use of Ag-Cs NPs at the three concentrations evaluated did not present a toxic effect after application every 7 days for 30 days in tomato seedlings. In addition, the earthworm humus with the three concentrations of the nanoparticles favored the height, the diameter of the stem and the dry and fresh weight of the seedlings biomass in comparison with the control seedlings, emphasizing this effect at the concentration of 2000 ppm.
Effect of NPs of Ag-Cs on the development of vascular wilt in tomato seedlings inoculated with Fusarium oxysporum
The tomato seedlings were infected with F. oxysporum, observing wilt and yellowing symptoms after 14 days of inoculation (Figure 5). On the other hand, control seedlings without inoculation and those treated only with Ag-Cs NPs had a higher number of leaves and a bright green color com-pared to infected seedlings. The group treated with Ag-Cs NPs, managed to reduce the severity of the disease with as-pect very similar to control group, after 5 days of inoculation.

Figure 5 Tomato seedlings after 14 days in an incubation chamber at 25 °C. A) uninoculated
seedlings, B) seedlings inoculated with Fusarium
oxysporum, C) uninoculated seedlings treated with
Ags-CS NPs, D) seedlings inoculated and treated with 2000 ppm AgNPs-CS.
Figura 5. Plántulas de tomate durante 14 días en una
cámara de incubación a 25°C. A) Plántulas sin infectar, B) Plántulas
infectadas con Fusarium oxysporum, C) Plántulas sin infectar
tratadas con nanopartículas de plata recubiertas de quitosano
(AgNPs-CS), D) Plántulas infectadas y tratadas con 2000 ppm de
AgNPs-CS.
Figure 6 shows the height and diameter of the stem of the tomato seedlings with the different treatments. The development of the Ag-Cs NPs treated seedlings, after 5 days of inoculation, did not affect the height and diameter of the plants stem (p˃0.05), since no significant difference was observed against the control seedlings. Therefore, the treatment can be said did not work as a growth promoter, although it was not the objective of our study.

Figure 6 A) Height and B) diameter of tomato seedlings with different treatments during 14 days
of incubation at 25 °C 90% relative humidity.
Figura 6. A) Altura y B) diámetro de plántulas de
tomate con diferentes tratamientos durante 14 días de incubación a
25°C 90% de humedad relativa.
Figure 7 shows the dry weight of tomato seedlings 14 days after inoculation with a Fusarium oxysporum spores solution, and it can be seen that there were significant differences at the end of the experiment with respect to the treatment without application of Ag-Cs NPs (p˃0.05). On the other hand, it did not show differences regarding the control, demonstrating that the application of Ag-Cs NPs, for the control of phytopathogens such as F. oxysporum, could be an alternative to eradicate vascular wilt in tomato seedlings and it can work as a corrective treatment.

Figure 7 Dry weight of tomato seedlings (stem, root and leaf) exposed to the treatment of
silver nanoparticles coated with chitosan (2000 ppm). * indicates
significant differences between all treatments (p <0.05).
Figura 7. Peso seco de plántulas de tomate (tallo, raíz
y hoja) expuestas al tratamiento nanopartículas de plata recubiertas
de quitosano (2000 ppm). *indica diferencias significativas entre
todos los tratamientos (p <0.05).
Table 1 shows the enzymatic activity of catalase in tomato seedlings with the different treatments. With respect to time, the catalase activity increases with the days of incubation in most treatments. Compared to control, F. oxysporum fungus infected tomato seedlings and treated with Ag-Cs NPs 5 days after inoculation, showed higher catalase activity (130%), followed by tomato seedlings only inoculated (128%). The control seedlings without infection and without exposure to the nanoparticles showed the lowest catalase activity. Catalase is a key antioxidant enzyme in the plant’s defense system against pathogens; therefore, these results indicate that both inoculation and the nanoparticles induces the defense system of the plants.
Table 1 Activity of the catalase enzyme of tomato seedlings 14 days after
being exposed to the silver nanoparticles coated with chitosan.
Tabla 1. Actividad de la enzima catalasa de
plantas de tomate durante 14 días después de ser expuestas a las
nanopartículas de plata recubiertas de quitosano.
| Days | Treatments | Catalase Activity (U/mL) |
|---|---|---|
| 0 | Control | 12.22 ± 3.55 |
| 7 | 16.26 ± 2.19 | |
| 14 | 16.37 ± 2.05 | |
| 0 | Control Ag-CS NPs | 9.24 ± 0.05 |
| 7 | 15.83 ± 0.54 | |
| 14 | 16.92 ± 0.94 | |
| 0 | Control F. oxysporum | 12.49 ± 0.67 |
| 7 | 21.95 ± 4.10 | |
| 14 | 21.09 ± 1.21 | |
| 0 | 2000 ppm Ag-CS NPs | 13.72 ± 0.82 |
| 7 | 21.32 ± 7.08 | |
| 14 | 26.69 ± 3.83 |
Ag-Cs NPs showed significant antifungal activity in plant inoculation experiments and AgNP can bind and penetrate directly into the cell membrane to kill the fungus spores, although the AgNP penetration into the microbial cells membranes has not been fully understood (Ouda, 2014). Because the plants are of large foliar area and are of a stationary nature, they have a higher probability of exposure to a wide range of nanoparticles available in their environment (Anjum et al., 2013). The main route of plants exposure to nanoparticles is through the soil through leaching, direct application with deliberate release or from other products (Rajput et al., 2018).
The plants roots have been considered as the main exposure route of plants to NP, which can harbor a significant part of the accumulated nanoparticles, which may or may not produce physical or chemical toxicity in plant (Anjum et al., 2013). A study shows that shoots of Cucurbita pepo presented 4.7 more silver concentration in AgNP treated plants that those treated with a bulk solution at the same concentrations, considering a greater silver release from the nano-particles as the main reason (Stampoulis et al., 2009). Another study has demonstrated the uptake of AgNP through the roots of Arabidopsis thaliana, where although most of the nanoparticles adhere to the root boundary, their transport to shoots was also observed (Milewska-Hendel et al., 2019). The possible mechanism of nanoparticles absorption from plants can be through primary roots or laterial roots. Then, they are transported from the root through the stem to the leaf. They could also be adsorbed on the surface of the roots (Tripathi et al., 2017). A study on the uptake of AgNP and Ag+ in rice plants, observed a higher silver uptake when it was in NP, compared with that of Ag+, this can be explained due the formation of AgCl on the root surface reducing the bioavailability and translocation of Ag and the direct internalization of AgNPs by plant (Yang et al., 2020).
There are few studies that evaluate the effect of metallic nanoparticles in plants. It has been previously reported that AgNP were effective in reducing 50% of the phytopathogens Bipolaris sorokiniana and Magnaporthe grisea growth on perennial ryegrass (Lolium perenne) (Jo et al., 2009). These authors conclude that the effectiveness of nanoparticles is greater with a preventive application due to one that promotes the direct contact of silver with spores and germ tubes to inhibit its viability. In our study, we first inoculated, and then applied the treatment, which would be interesting to evaluate the effect of the nanoparticles in a preventive manner. In a more recent study, Nejad et al. (2016) were able to control disease progress of rice plants inoculated with Rhizotocnia solani using AgNP in a concentration-dependent manner, this investigation suggests AgNP can replace chemical pesticides in controlling and inhibiting sheath blight, a common disease in rice.
Conclusion
In this study silver nanoparticles coated with chitosan were synthesized with the purpose of being used as control for vascular wilt disease caused by F. oxysporum in tomato seedlings. The NPs showed antifungal activity in vitro and did not affect the normal growth of the plant or show any toxic effect in the tomato seedlings. In addition, the synthesized nanoparticles were effective in eradication of the disease caused by F. oxysporum. Our results indicate that nanotechnology is a promising strategy for the control of phytopathogens in agricultural crops.











 nueva página del texto (beta)
nueva página del texto (beta)



