Introduction
The main threats to population stability and health of marine fishes are overexploitation, habitat degradation, pollution (Dulvy et al., 2003) and exotic species. Fishing affects the age structure of fish populations, including age truncation, even at low fishing mortality levels (Berkeley et al., 2004; Hsieh et al., 2010). Nevertheless, overexploitation of fisheries might induce local extirpations, thus contracting the range of the species (Dulvy et al., 2000). As a result, some fishery resources have been listed as endangered, but the validity of listing in the case of fish is still a subject of debate (Powles et al., 2000; Del Monte -Luna et al., 2008). Geographic distribution is a key factor for critically endangered species when abundance data are not available, since species that have recovered show the same or greater range after a perturbation (Lotze et al., 2011; IUCN, 2012). Hence, population age structure and range may be used as a proxy for population health status when additional data are not available.
Totoaba (Totoaba macdonaldi; Gilbert, 1890) is a critically endangered species, endemic to the Gulf of California, with a distribution range from the mouth of the Colorado River to Bahía Concepción in the west coast and until El Fuerte River (Las Grullas, Sinaloa) in the east coast of the Gulf of California. Totoaba fishery promoted the development of the three main fishing communities in the Upper Gulf of California, i.e., San Felipe, Baja California, Golfo de Santa Clara, and Puerto Peñasco, Sonora. Fishing first aimed at the consumption of its swim bladder (maw), and later of its meat (Flanagan and Hendrickson, 1976). The sharp reduction in totoaba catches caused by overfishing, habitat degradation, and juvenile bycatch by the shrimp fleet evidenced a steep decline in population abundance at an alarming rate (Flanagan and Hendrickson, 1976; Cisneros-Mata et al., 1995). This situation triggered an indefinite ban since 1975 (DOF, 1975). The following year, the Convention on International Trade in Endangered Species (CITES) included totoaba as the first marine fish in danger of extinction. In 1979, the United States Fish and Wildlife Service (FWS) added it to the list of endangered species. Currently, this species is included as critically endangered in the Red List of Threatened Species of the International Union for the Conservation of Nature (IUCN) (Version 2018-1, https://www.iucnredlist.org). In Mexico, it is still listed as “in danger of extinction” in NOM-059-SEMARNAT-2010 (DOF, 2010).
In recent years, totoaba poaching has increased because of the high price of its swimming bladder in the Asian market (Valenzuela-Quiñonez et al., 2014; Greenpeace East Asia, 2015). There is a growing social pressure demanding the government to withdraw the current permanent ban of the totoaba (Valenzuela-Quiñonez et al., 2011; García-De León, 2013). However, its current classification as an endangered species has limited studies on its biology and population status (Valenzuela-Quiñonez et al., 2011; De Anda-Montañez et al., 2013; García-De León, 2013). Some studies have focused on age and growth (Nakashima, 1916; Berdegué, 1955; Flanagan, 1973; Molina-Valdéz et al., 1988; Román-Rodríguez and Hammann, 1997; Pedrín-Osuna et al., 2001), or the relationship between the ecological factors associated with the lower catches (Lercari and Chávez, 2007). Rowell et al. (2008a), provide evidence that river diversion can have a dramatic effect by slowing growth during the juvenile stages, thus delaying maturation. Rowell et al. (2008b) validate the relationship between temperature, water oxygen isotope composition (δ18O) and otolith δ18O. Other studies focus on the distribution of juveniles and its relationship with salinity (Valdez-Muñoz et al., 2010), the rate of rebound estimate for totoaba (r2M = 0.055) (Márquez-Farías and Rosales-Juárez, 2013), and recently, genetic diversity and population dynamics (Valenzuela-Quiñonez et al., 2014, 2015, 2016). Never-theless, some basic questions remain valid, such as which is the distribution, age structure and individual growth of this species today? These have not been answered after many years of being a species still considered as critically in danger of extinction.
Distribution is the second most important criteria, after abundance, to identify threatened categories (IUCN, 2012), and totoaba might show changes in its range along with a shortened age structure, as expected for a threatened species. In this paper, we reviewed surveys from 2010 to 2014 with the following objectives, (1) to explore the distribution area of T. macdonaldi in the Gulf of California to test the hypothesis of contraction of the distribution range, and (2) to determine the spatial distribution of the different age groups and individual growth pattern.
Material and methods
Research licenses
The research and scientific collection was done following the research protocol described in the project entitled “Health and Conservation status of the totoaba population (T. macdonaldi) in the Gulf of California: a critically endangered species” (CONABIO: FB1508/HK050/10; CONACYT 2011-01/165376). These studies were promoted, endorsed and financed by various government institutions in Mexico (“Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, CONABIO; Comisión Nacional de Áreas Naturales Protegidas, CONANP; Dirección General de Vida Silvestre of Secretaría de Medio Ambiente y Recursos Naturales, SEMARNAT; and Consejo Nacional de Ciencia y Tecnología, CONACYT”). Besides, the Mexican government issued the scientific collection permits (SGPA/DGVS/02913/10, SGPA/DGVS/05508/11, SGPA/DGVS/00039/13, and SGPA/DGVS/00230/14) based on the above-mentioned research protocol. We work always meeting the demands, requirements and protocols that are required by the various institutions, and always conducting our research with responsibility, ethics and professionalism, particularly in relation to this study that involves a critically endangered species.
Sample collection
From April 2010 to July 2014, a total of 20 sampling trips were carried out at 12 sites along the Gulf of California, indicated with the number of individuals captured; Upper Gulf of California and Colorado River Delta Biosphere Reserve (n = 204), Piedra Consag (n = 70), South of San Felipe (n = 15), San Luis Gonzaga (n = 86), Ensenada Grande (n = 30), Bahía de Los Ángeles (n = 2), Bahía Concepción (n = 1), Bahía de La Paz (n = 24), El Desemboque (n = 6), Bahía de Lobos (n = 21), Las Grullas (n = 1), and Mazatlán, Sinaloa (n = 2) (Fig. 1), using 26-feet boats with outboard motor. The fishing gear used depended on the depth at the collection site: in Piedra Consag and San Luis Gonzaga, with depths between 30 and 60 meters, a hook and line were used; in sites with depths between 2 and 38 meters, a gillnet measuring 120 m in length with a mesh size of 10 inches (25.4 cm) was used. A random sampling was performed, with a higher sampling frequency in Upper Gulf sites, which is reflected in sample size (Table 1). For each organism collected, we recorded its geographical position, total length (cm), body weight (kg), and sex; both otoliths were extracted from each individual. Otolith samples from different years (2010-2014) and areas of the Gulf of California were combined in a database of a single year (“synthetic year”), assuming that interannual growth pattern was unchanged.

Figura 1. Distribución espacial histórica de Totoaba macdonaldi en el Golfo de California (líneas grises marcadas en el mapa). Sitios de muestreo, distribución y estructura de edad de totoaba durante el periodo de muestreo 2010-2014.
Figure 1 Historical spatial distribution of Totoaba macdonaldi in the Gulf of California (grey lines marked on the map). Sampling sites, distribution, and age structure of totoaba during the 2010-2014 sampling period.
Table 1 Sampling sites, overall sample size (N), and sample size (n) to cal-culate the sex
ratio of Totoaba macdonaldi in the Gulf of
California.
Tabla 1. Sitios de muestreo,
tamaño total de muestra (N) y tamaño de muestra (n) para calcular la
proporción de sexos de Totoaba macdonaldi en el Golfo de
California.
| Sampling sites | N | n |
Sex ratio (F:M) |
X2 |
| Biosphere Reserve | 204 | 186 | 0.74:1 | 4.22* |
| Piedra Consag | 70 | 53 | 1.21:1 | 0.47 |
| South of San Felipe | 15 | 13 | 1.17:1 | 0.08 |
| Las Encantadas | 86 | 46 | 1.19:1 | 0.35 |
| Ensenada Grande | 30 | 0 | - | - |
| Bahía de Los Ángeles | 2 | 0 | - | - |
| Bahía Concepción | 1 | 0 | - | - |
| Bahía de La Paz | 24 | 9 | 3.50:1 | 2.78 |
| El Desemboque | 6 | 3 | - | - |
| Bahía de Lobos | 21 | 18 | 0.50:1 | 2.00 |
| Las Grullas | 1 | 1 | - | - |
| Mazatlán | 2 | 2 | - | - |
| Total | 462 | 331 | 0.89:1 | 1.48 |
Critical value X2 (α = 0.05, 1) = 3.84; *significant differences (p < 0.05).
Age structure: by sex and locality
In the laboratory, annual growth rings in sectioned otoliths were read to determine age following the technique described by Beckman et al. (1990) and Lowerre -Barbieri et al. (1994), as described in Román-Rodríguez and Hammann (1997). Three readings per otolith were performed under a stereomicroscope (Zeiss Stemic 2000- C, Göttingen, Germany). Thus, the age of each individual was defined as the average count of the readings. To avoid bias in the readings, when the difference between readers was > 5 %, the otolith was read again. The index of average percent error (IAPE) and the coefficient of variation (CV) (Campanaet al., 1995) were calculated to assess the reliability of the reading counts between readers; this means that these quantitative indices measure the consistency among determinations. According to Campana (2001), ageing error can be expressed as follows: precision, defined as the discrepancies on reproducibility of repeated measurements on a given structure; and accuracy, denoting the differences between the closeness of the age estimate to the true value. The reliability of the reading was accepted when IAPE and CV were less than 5 % and were estimated as follows (Campana, 2001):
where N is the number of individuals aged, R is the number of readings, X ij is the i th age determination of the j th fish, and X j is the mean age of the j th fish (Campana, 2001).
The validation of annual deposition of growth rings was confirmed by Román-Rodríguez and Hammann (1997). These authors caught three juveniles of T. macdonaldi with ages less than one year, and the organisms were kept alive in captivity; thus, total length (mm) and total weight (g) data were taken every month beginning five months after capture. The age validation was done when the fishes died 12 and 24 months later. According to Campana (2001), this procedure contains a high scientific value to confirm and support the accuracy of age interpretations; it is recommended for annual or daily growth increments and is applicable to any age range; the main advantages are simultaneous validation of both absolute age and periodicity of growth structures, the procedure has very high precision (± 0 year), and the sample size is minimum (> 1 specimen). Thus, Román-Rodríguez and Hammann (1997) used the best method to validate the growth increments in T. macdonaldi. After estimating age for all specimens, we constructed a distribution map of positive catches (scientific collection) in the Gulf of California with georeferenced observations and age-frequency histograms.
Growth
The asymptotic growth of totoaba (T. macdonaldi) was analyzed based on the von Bertalanffy growth model (von Bertalanffy, 1938). The model was proposed considering the biological principles of individual growth and suitably describes properties of growth curves such as inflection point and asymptotic limit. Thus, otoliths of 424 individuals (178 females, 195 males, and 51 undifferentiated) were collected for age determination. Measurements of total length (TL) and total weight (TW) were taken to the nearest 0.1 cm and 0.1 g, respectively. The von Bertalanffy growth model is expressed as follows:
were L t is the estimated total length; t represents time (year); L∞ is average maximum total length reached by older individuals; k represents the growth coefficient;t 0 parameter is the theoretical age of the individuals at zero size under the assumption that the von Bertalanffy growth curve describes the growth accurately right down to zero length.
Even if this unlikely assumption is true, fish will be born with some positive length, so t 0 will usually be negative. Hereafter, the parameters in the growth model (L ∞ , k, t 0 ) will be defined as θ i parameters, which were estimated based on a Bayesian approach. The most important advantage of Bayesian methods is that they provide a framework to assign probabilities to different hypotheses using resource observation information and inferences based on other stocks/species. In the Bayesian method, posterior density functions of model parameters (θ i ) are derived based on the goodness-of-fit of a model to the dataset and on prior information not contained in those data. The posterior probability distributions p(θ i │data) of a set of continuous model parameter (θ i ) given the data are determined using Bayes’ theorem (Berger, 1985):
where p(θ i │data) denotes the joint probability for a set of parameters θ i and the obtaining of the data. Suppose that the data represent n observations of a continuous probability density function (PDF) that depends on the unknown parameters θ i , in a known way that can be described by the von Bertalanffy growth model (Siegfried and Sansó, 2006; He and Bence, 2007; Alós et al., 2010). Thus, p(θ i ) describes the prior PDF (commonly called “prior probability”) for θ i , and L(data│θ i ) is the probability of obtaining the data values if θ i were the true values; in this study, L(data│θ i ) is referred to the likelihood function describing the dependence of the data on θ i , assuming that the likelihood function of the entire data set is given by the product of the normal density function over all data points; the function is expressed as follows:
where σ is the standard deviation of the observation error (Chen and Fournier, 1999; He and Bence, 2007). Accor-ding to Punt and Hilborn (1997), the prior distribution for the θ i parameters must be based on previous knowledge of the parameter, without incorporating the data used to calculate the likelihood function. Priors are usually obtained by consul-ting experts or historical records; however, T. macdonaldi has very limited information about the demography and basic biology (e.g. growth, reproduction).
The most recent study analyzing age and growth for T. macdonaldi was documented twenty-two years ago (Román-Rodríguez and Hammann, 1997). Previously, using scales and identifying growth rings, the growth pattern of this species was described by Nakashima (1916), Berdegué (1955), Flanagan (1973), and Molina-Valdéz et al. (1988). Basically, since 1916 only five studies on T. macdonaldi age and growth have been reported; consequently there are many uncertainties regarding growth parameters. Thus, prior distributions of each of the parameters were constructed independently, as-suming a non informative Bayesian priors based on uniform distribution as follows: k=U(0.14,0.75), t 0 =U(-3.29,-0.49), and L ∞ =U(143,200). These priors were assumed independent of each other and of both fish length and age.
A prior can be either informative or non-informative. A non-informative prior provides little information relative to the data and usually is either a uniform distribution or one with a large variance, giving all reasonable parameter values approximately equal probabilities. The Bayesian analysis was implemented using JAGS (Just Another Gibbs Sampler) version 4.0.0 (Plummer, 2015) using the run jags library within R software (R Core Team, 2015), with 100,000 iterations in three chains using thinning of 10, dropping the first 15,000 iterations as burn-in and therefore leaving 25,500 samples per chain in the analysis. The initial values of chains were drawn randomly from priors previously described. The growth parameters for females, males, and whole population were estimated following the Bayesian approach. Thus, to compare the fits achieved by each data set, the deviance information criterion (DIC) was used. A smaller value of DIC corresponds to a better fit (Gelman et al., 2004).
Biometric relationships
Total length (cm) and total weight (kg) data were adjusted to a power regression model, TL=aWb, where a is the condition factor (changes related to weight) and b is the adjustment parameter (growth type). The “F” test was applied to the model and the “Student t” test was applied to b values under the hypothesis that b = 3 (isometric growth) (Sparre and Vennema, 1997). Length-weight data were pooled across sample sites, as sample size was small for some loca-lities (Table 1); in addition, all specimens were considered as belonging to a single population in the Gulf of California (Valenzuela-Quiñonez et al., 2016).
Sex ratio
Organisms were sexed by visual analysis of gonad differentiation, and sex assignment in individuals with non-differentiated gonads by standard histological techniques (Humason, 1979), and the sex ratio per sampling site was assessed by a chi-squared test (X2) at a significance level of α =0.05 (Table 1). It is assumed that the sex ratio is 1:1 (females (F):males (M)), and that the type of sampling gear catches the two sexes at random, hence not influencing the sex ratio.
Results
Distribution
A total of 460 totoaba individuals were collected in the Gulf of California. The presence of this species was recorded along the continental coast from the Golfo de Santa Clara, Sonora to Mazatlán, Sinaloa. Likewise, totoaba was observed along the coastline from the Colorado River Delta to Bahía de La Paz, suggesting a potential increase of its known records in the southern distribution limit down to Bahía de La Paz, Baja California Sur, and Mazatlán, Sinaloa (Fig. 1). The largest number of organisms collected was recorded in the area of the Upper Gulf of California and Colorado River Delta Bio-sphere Reserve; this is the area where totoaba are most easily sampled, as it aggregates to spawn. The other two areas with high abundances were the islands known as Las Encantadas close to Bahía San Luis Gonzaga, and Piedra Consag off the coast of San Felipe, Baja California. Low numbers of organ-isms were obtained in other localities (Table 1; Fig. 1).
Overall age structure: by sex and locality
The IAPE and CV values for all individuals combined were 1.54% and 2.01%, respectively, indicating high consis-tency among readings. Figure 2 shows the annual growth rings in a sectioned otolith of an individual estimated to be 8 years old. Totoaba population age structure in the Gulf of California is shown in Figure 3a. Age structure ranged from 0 to 19 years old, with the same age interval for females and males (Fig. 3b-c). The juvenile organisms (≤ 5 years) were dis-tributed throughout the Gulf of California, while adults were recorded only in the Upper Gulf area, from the Midriff archipelago region (Ángel de la Guarda and Tiburón islands) and northward (Fig. 1). The sample corresponding to Ensenada Grande consisted of corpses found lying on the beach and included adults only; one 24 year-old individual was observed, but the sex could not be determined because it had been caught previously and the internal organs were removed by poachers (Fig. 1). The more abundant age groups were from 1 to 8 years. Although the abundance of individuals for the age groups from 9 to 19 years diminished for both sexes; the females showed more abundance of age-at-length data for individuals older than 9 years in comparison to males.
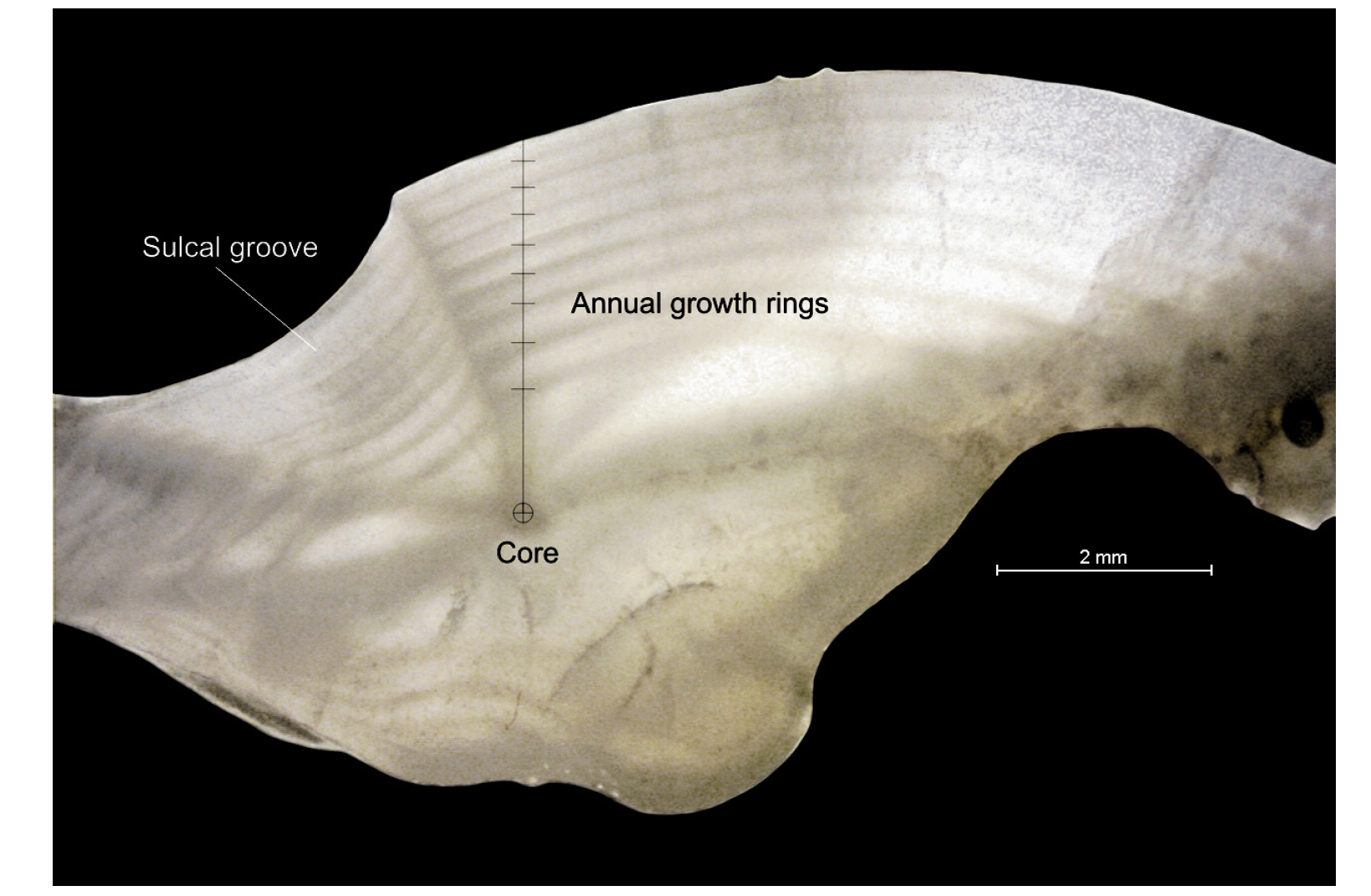
Figura 2. Fotografía de la sección transversal de un otolito de totoaba adulta que muestra los anillos de crecimiento anual, con una edad estimada de 8 años.
Figure 2 Photograph of transverse section of an adult totoaba otolith showing the annual growth rings with an estimated age of 8 years old.
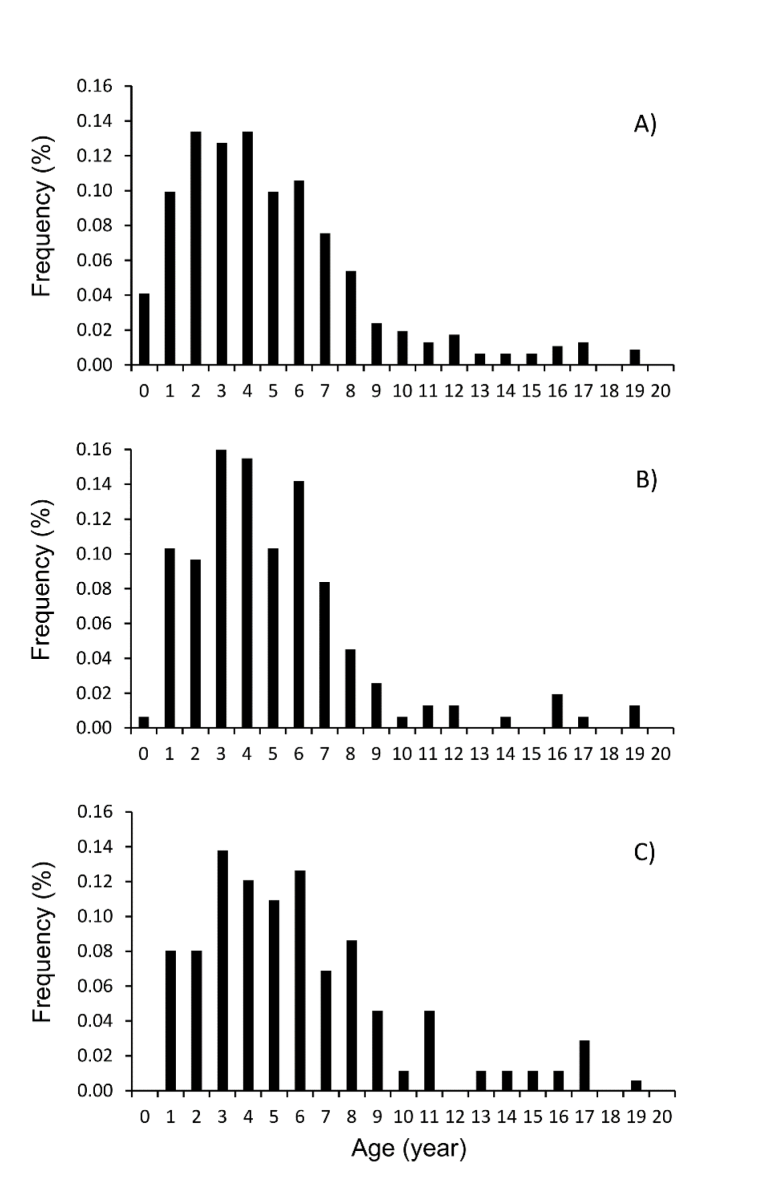
Figura 3. Estructura de edad. A) Datos generales, incluyendo individuos no diferenciados; B) Hembras; y C) Machos de Totoaba macdonaldi durante el período de muestreo 2010-2014 en el Golfo de California.
Figure 3 Age structure. A) Overall data, including undifferentiated individ-uals; B) Females; and C) Males of Totoaba macdonaldi during the 2010-2014 sampling period in the Gulf of California.
Growth
The growth analysis based on the Bayesian approach showed very high performance for fitting the von Bertalanffy growth model to females, males and the full population of T. macdonaldi; the growth parameters thus estimated are shown in table 2. There were differences among estimates of L∞; this value showed that females (165.25 cm ) were larger than males (154.50 cm), whilst estimates of the theoretical age∼ of the individuals at zero size were similar among them (t 0 -0.48).
Table 2 Von Bertalanffy Growth parameters estimated for Totoaba macdonaldi in
the Gulf of California.
Tabla 2. Parámetros de
crecimiento de von Bertalanffy estimados para Totoaba macdonaldi en
el Golfo de California.
| Sex | N | K (sd) | L∞ (sd) | to (sd) | DIC |
|---|---|---|---|---|---|
| F,M,U | 424 | 0.27 (0.003) | 164.72 (0.22) | -0.49 (0.008) | 5242.70 |
| Female | 178 | 0.28 (0.006) | 165.25 (0.73) | -0.48 (0.200) | 2212.40 |
| Male | 195 | 0.31 (0.006) | 154.50 (0.48) | -0.48 (0.020) | 2344.70 |
F; female, M; male, U; undifferentiated, n; sample size, sd; standard deviation, DIC; deviance information criterion.
The growth coefficient had a slight variability, between 0.27 and 0.31 for the entire population and males, respectively. The theoretical growth curves fitted to the different observed age-at-length data sets are shown in figures 4a, b, and c. Given that the posterior distributions (Bayesian outputs) of the growth parameters were estimated by Markov chain Monte Carlo simulation, the performance of the three chains in the simulation process showed high convergence for all the θ i growth parameters including the standard deviation (Fig. 5 A-L). Although the three growth models were suitable, the variability in the θ i parameters cannot be ignored; conse-quently, the deviance information criterion showed that the theoretical growth estimated for females was the best fit (DIC = 2212.4, Table 2). Comparatively, the growth model for the whole population had the greater expected predictive error (DIC = 5242.7, Table 2).
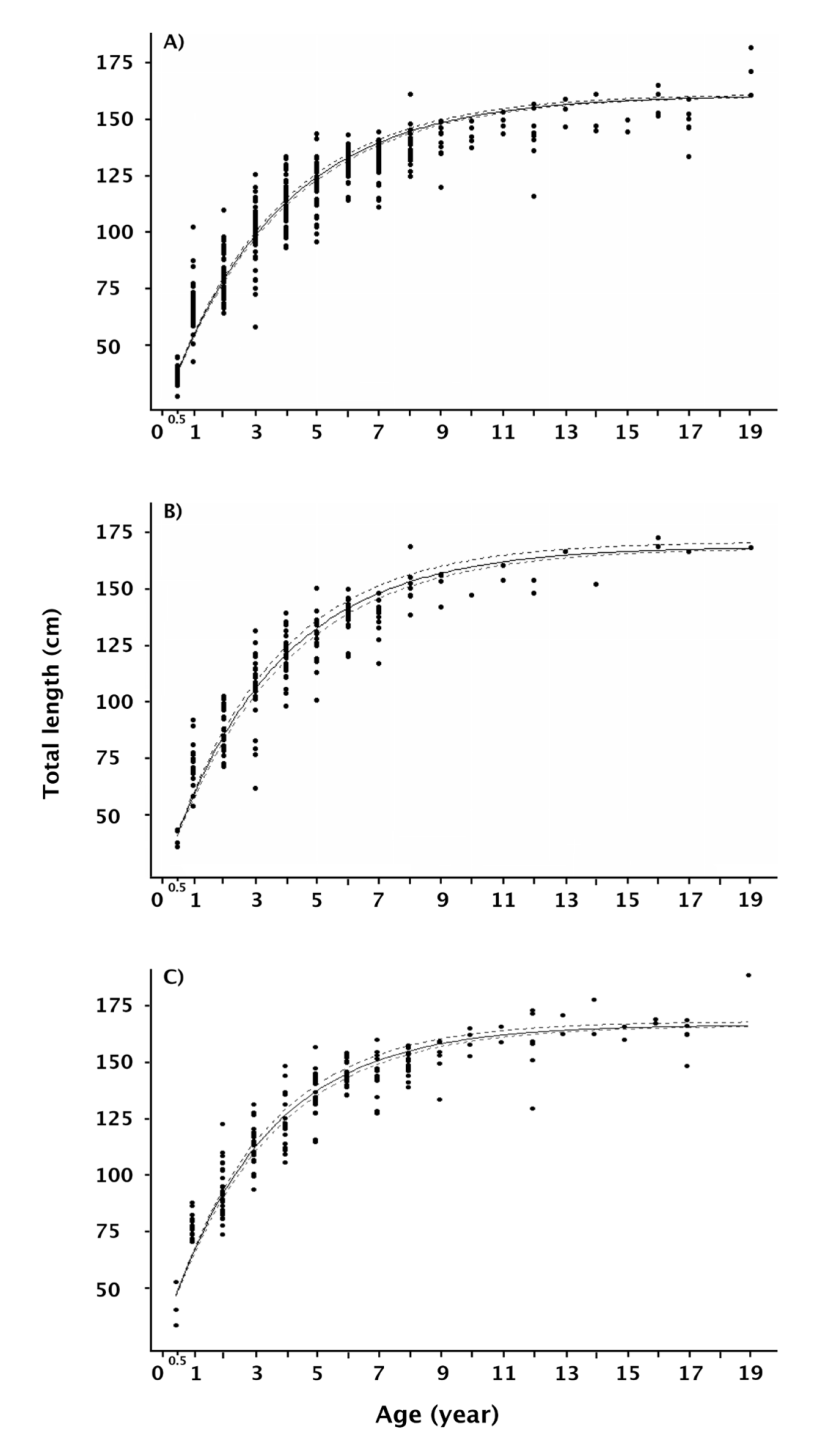
Figura 4. Modelo de crecimiento de von Bertalanffy ajustado a datos de talla-edad de Totoaba macdonaldi. A) Población total (hembras, ma
Figure 4 Von Bertalanffy growth model fitted to size-at-age data ofTotoa-ba macdonaldi. A) Whole population (female, male and undifferentiated); B) Female population; and C) Male population.

Figura 5. Distribuciones posteriores (iteraciones en tres cadenas) para los parámetros de crecimiento de von Bertalanffy (k, L∞, to and sd) de Totoaba macdonaldi en el Golfo de California. A-D) Población total (hembras, machos e indiferenciados); E-H) Población hembras; e I-L) Población machos.
Figure 5 Posterior distributions (iterations in three chains) for the von Bertalanffy growth parameters (k, L∞, t o and sd) of Totoaba macdonaldi in the Gulf of California. A-D) Total population (female, male and undifferentiated); E-H) Female population; and I-L) Male population.
Biometric relationships
Total length-total weight relationship for the entire population and by sex are shown in figures 6a, b, and c. The F-test applied to the slope in the regression models were highly significant (p = 0.00), and the Student t test applied to b values (with standard errors of 0.017, 0.043, 0.038 for the whole population, females and males, respectively) showed no significant differences (p > 0.05), suggesting an isometric growth (b = 3) for the entire population, females and males.
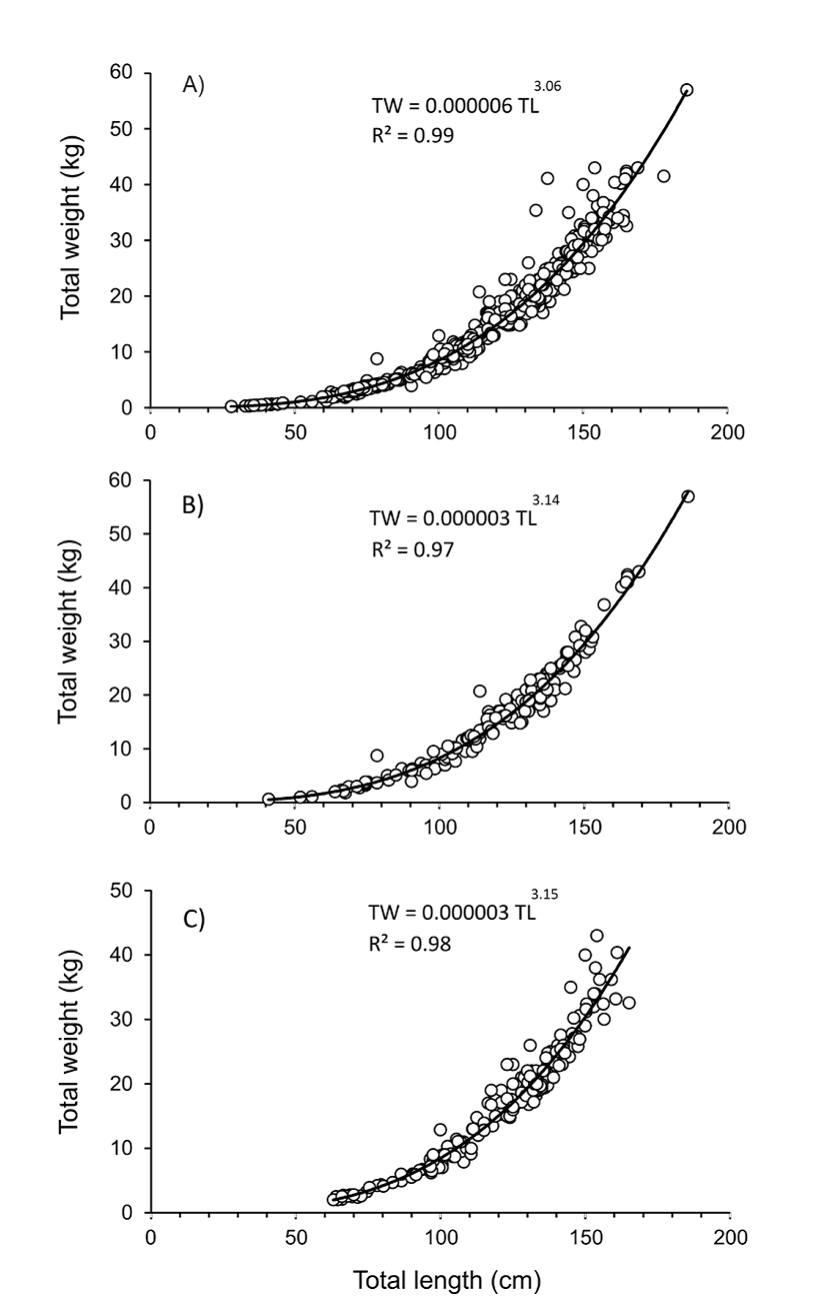
Figura 6. Relación longitud-peso de Totoaba macdonaldi durante el período de muestreo 2010-2014 en el Golfo de California. A) Datos generales, incluyendo individuos no diferenciados; B) Hembras; y C) Machos.
Figure 6 Length-weight relationship of Totoaba macdonaldi during the 2010-2014 sampling period in the Gulf of California. A) Overall data, including undifferentiated individuals; B) Females; and C) Males.
Sex ratio
The sex ratio varied across sample sites with no ap-parent latitudinal or spatial gradient; and was not different from the expected 1:1 (F:M) ratio (X2 =1.48, p > 0.05) (Table 1). Only in the area of the Upper Gulf of California and Colorado River Delta Biosphere Reserve males were significantly more numerous than females (X2 = 4.22, p < 0.05) (Table 1). When the data of the Biosphere Reserve were analyzed by date, the sex ratio varied over time, but did not show significant differ-ences (p > 0.05), except for data recorded during March/2013 (p < 0.05) (Table 3). Although there are sites and sampling dates with n less than 30 individuals, in which the results of the Chi-square test could be questioned, it is clear that the sex ratio of the totoaba population in the Gulf of California remains 1:1.
Table 3 Sampling dates for Biosphere Reserve and sample size (n) to calcu-late the sex ratio
of Totoaba macdonaldi in the Gulf of California.
Tabla
3. Fechas de muestreo para la Reserva de la Biosfera y
tamaño de muestra (n) para calcular la proporción de sexos de
Totoaba macdonaldi en el Golfo de California.
| Sampling dates | n | Sex ratio (F:M) |
X2 |
|---|---|---|---|
| February/2011 | 33 | 0.74:1 | 0.76 |
| April/2012 | 13 | 1.6:1 | 0.69 |
| January/2013 | 62 | 1.2:1 | 0.58 |
| February/2013 | 18 | 0.8:1 | 0.22 |
| March/2013 | 45 | 0.2:1 | 18.69* |
| April/2013 | 6 | 1.0:1 | 0.00 |
Critical value X2 (α = 0.05, 1) = 3.84; *significant differences (p < 0.05).
Discussion
One of the potential impacts of fishing on the species that leads to population collapse is the reduction of its distribution areas (Dulvy et al., 2000; 2003). Historical records of totoaba distribution date back to the early 20th century, stating from the mouth of the Colorado River to the Fuerte river (Las Grullas, Sinaloa) on the mainland, and south to Bahía Concepción off the peninsular coast (Arvizu and Chávez, 1972; Robertson and Allen, 2015). Totoaba, as a critically endangered species might suffer a contraction of its distribution range. However, this study suggests that the historical distribution range of totoaba is not only maintained, but may have even expanded, both along the coast to Bahía de La Paz and along the continental coast to Mazatlán, Sinaloa. Despite the possible scenario, the current assessment of the spatial presence along the gulf supports the idea that the collapse of the totoaba fishery did not result in a contracted distribution range. Alternatively, if this occurred, it could have expanded again after approximately 40 years of closed fishing season, even considering the incipient illegal fishing that occurred in the Upper Gulf of California during this period.
The species was apparently resilient to the anthro-pogenic pressure exerted on its population forty years ago. However, Márquez-Farías and Rosales-Juárez (2013) considered that totoaba showed low resilience to the fishing pressure, mainly due to the migratory and long-life characteristics of this endemic species. In the sampling sites of this study, poaching on the totoaba population in the Upper Gulf during the breeding season was observed and continued through the summer months in the area of the larger islands. This, because of the high demand for the swim bladder in the Chinese black market (Greenpeace East Asia, 2015), representing a high risk factor for the species.
Fish populations subjected to heavy exploitation tend to show a shortened age structure due to the removal of older organisms from the population (Hsieh et al., 2010). Totoaba showed a wide range of ages from 0 to 24 years, and the maximum length observed, 1860 mm, is similar to the maximum sizes reported prior to the population collapse (1830-1960 mm; Nakashima, 1916), as well as 16 years after the moratorium was established (Román-Rodríguez and Hammann, 1997). Nevertheless, the maximum size and age composition of the population at the beginning of the fishery is currently unknown. In this study, it is clear that the age distribution of the fish sampled in the Biosphere Reserve region (Upper Gulf) is the best representation for the totoaba population, showing ages spanning from 0 to 19 years. When the data were pooled, since totoaba is considered to be a panmictic population from the Gulf of California (Valenzuela- Quiñonez et al., 2016), a truncated population showing the absence of some adult age classes and biased towards juveniles was observed. This may indicate the impact of fishing pressure years ago, but may also reflect the limitations of sampling, associated with the difficulty to access recruitment areas, and to the small sample size (special care was taken in this regard given the conservation status of the species). In this study, the age structure of the totoaba population was similar to that reported before its collapse (Nakashima, 1916), and could be considered as a population stability marker, as mentioned by Kloser et al. (2015). However, the poaching increase from 2013 to date, mainly in the Upper Gulf and the Midriff archipelago region, may have already affected the population structure.
Adult organisms were collected mainly in the Upper Gulf area, while juveniles were widely dispersed across the Gulf of California. This spatial pattern had been inferred from following migratory movements of totoaba along the coast (Arvizu and Chávez, 1972; Flanagan and Hendrickson, 1976; Cisneros-Mata et al., 1995). Nevertheless, no precise records on the spatial variation of age composition were available. Adult organisms were found only in Upper Gulf of California towards the Midriff archipelago region, and they were absent southward of this region; this does not necessary indicate a real absence of adults, but might be a sampling bias and/ or that adults were not vulnerable to the fishing gears employed in the sampling season. Two main recruitment areas were identified: a) Piedra Consag off the coast of San Felipe in Baja California, and b) Las Encantadas islands (north of Bahía San Luis Gonzaga). During all sampling events, juvenile organisms, particularly between 1 and 3 years old, were collected throughout the year in both areas.
This study suggests an expansion of the totoaba population in the Gulf of California, Mexico, mainly by registering scientific collection of juveniles in La Paz, Baja California Sur, and Mazatlán, Sinaloa, including the presence of juveniles of similar ages collected from several localities during the same season (winter). Currently, there is a totoaba farm in the Bahía de La Paz, and the first fingerlings arrived in August 2012, which could cast doubt on the origin of the totoabas captured in this study during August and September 2013. However, the age structure of these 24 organisms registered in the Bahía de La Paz showed a range of 2 to 6 years old (Fig. 1), above the age of organisms from the farm which would be 1 year old. Therefore, catches within this age range (2-6 years) suggest new record of totoaba in Bahía de La Paz. Considering this new finding, it is now necessary to reconsider the current knowledge on T. macdonaldi distribution and migration to determine the movements of organisms on both coasts of the Gulf of California, as well as the causes and effects associated.
During 1975, when this fishery resource was classified as an endangered species, the Mexican government declared a moratorium on fishing totoaba. Thus, the access to biological and statistical data was limited, and several demographic studies were delayed; consequently, the most recent age and growth data for modeling theoretical growth curves of T. macdonaldi were collected during 1986-1991 (Román-Rodríguez and Hamman, 1997). They reported L ∞ = 135.5 cm (fork length) for the entire population, while the new data showed a larger average maximum total length (164.7 cm total length), including females (165.2 cm total length); even the male population showed a larger L∞ (154.5 cm total length). This study indicated that males are smaller than females, although both have similar growth coefficients and theoretical age of the individuals at zero size. The growth coefficient values estimated in this study were similar to those reported by Román-Rodríguez and Hamman (1997), indicating that the growth coefficient has had low variability along time, varying between 0.27 and 0.32 year-1; except for the growth coefficient estimated by Flanagan (1973) with a value of 0.16 year-1 obtained from growth ring counted in scales. About t0 parameter, the estimations showed uncer-tainty, varying from -0.05 (otoliths) to -2.26 (scales). Given the limited biological information on T. macdonaldi, the Bayesian approach uses the information contained in the age and length data, and the prior information on growth parameters. Thus, the deviance information criterion showed that the growth studies in this species must be explicitly by sex. The female growth model corresponded to a better fit, whilst the growth model for the entire population had a greater expected predictive error.
The main effect was observed in L∞, the difference between females and the whole population was only 1 cm total length, while the difference between males and the entire population was almost 10 cm total length. The length frequency distribution analyzed by Román-Rodríguez and Hamman (1997) had two modal groups, juveniles between 10 and 35 cm, and adults between 120 and 155 cm, the length structure between 50 and 110 cm was absent in the biological samples, approximately individuals from 5 to 10 years were not included in the analysis. Therefore, the uncertainty in the growth parameters estimated by Román-Rodríguez and Hamman (1997) corresponded to the challenge of fitting a growth curve from two sources of data, 1,125 juveniles smaller than 35 cm fork length, and 157 adults larger than 120 cm fork length. Based on these data, they explained that the biggest increment in fork length occurred during the first and second year, and a notable decrease in length was observed when totoaba reaches the age at first maturity, approximately between six and seven years. In contrast, our study shows a slower growth pattern, and between the first and sixth year the increment in length is faster than the growth observed for individuals seven years and older.
In the current study, the age and length structure included all age groups showed a low frequency of individuals older than 10 years, assuming that the age and length data and Bayesian priors for the parameters based on uniform distribution were jointly informative on the θ i parameters estimated for the von Bertalanffy growth model, as was showed by the convergence of the three Markov chains estimated through this Bayesian analysis. The von Bertalanffy growth model was informative about the growth pattern of T. macdonaldi, inflexion points for early ages were not observed, and the presence of growth stanzas (abrupt changes in growth rates) were not evident throughout the life span of T. macdonaldi (otolith data). Consequently, the statistical confrontation among candidate growth models was not an issue to analyze in this study, although it could be a topic for future research in this species. The main advantage of von Bertalanffy growth model based on Bayesian approach was the comparison between two different age structures associated to the individual growth of T. macdonaldi during two periods, both identified as overexploitation and after declaration of a moratorium (almost 40 years).
T. macdonaldi was found to be a species with isometric growth (b = 3), confirming the findings of Román-Rodríguez and Hammann (1997). The sex ratio was 1:1; only the Upper Gulf Reserve, which is a totoaba spawning and breeding area, showed a higher proportion of males (Cisneros-Mata et al., 1995). Previous studies have reported that totoaba form schools with a higher proportion of males in the breeding season in the Upper Gulf (Arvizu and Chávez, 1972; Barrera-Guevara, 1992). The higher proportion of males in this locality might be related to a spatial variability in reproductive traits (Smith, 1978; Merrell, 1994). The reproductive behavior in sciaenids involves several males surround a female to ensure fertilizing the oocytes (Aalbers and Drawbridge, 2008); males may also compete for mating as a key process in sexual selection and mate availability (De Jong, 2011). Thus, this variability, with biases towards males over females, is common in sciaenid fishes in the spawning season (Shamsul and Ajazuddin, 1992; Aalbers and Drawbridge, 2008).
Conclusions
Actually, totoaba is a species still considered as critically in danger of extinction by several international and national organisms (CITES, IUCN, SEMARNAT); consequently, fishing was prohibited since 1975 by the Mexican govern-ment and so remains to date. Nevertheless, its exposure to poaching in the past six years jeopardizes the recovery of the species. Recently, Cisneros-Mata (2018) suggests that the totoaba population is well structured, both in size and age. However, the lack of baseline abundance data for the virgin population restrains comparisons for drawing solid conclusions regarding its current state relative to the previous population. Nonetheless, this study indicates that the totoaba population shows at least signs of population stability, showing an age structure with presence of individuals from 1 to 20 years, in comparison to 1986-1991 where individuals between 4 and 13 years were absent in the age structure of the population. Finally, establishing a sustainable fishing program for totoaba is still a challenging task given the current lack of information and biological knowledge of this species. Data on population abundance are required, as well as approaching fishing communities, particularly in the Upper Gulf, since the viability of any management measures will largely depend on social acceptance. Thus, decision-makers are encouraged to implement an effective strategy to avoid illegal fishing, which has not been eradicated since January 2013 to date, and to conduct rigorous scientific assessments aimed to establishing management and/or conservation strategies for totoaba in the Gulf of California.











 text new page (beta)
text new page (beta)



