Introduction
Xylitol (C5H12O5) is a five-carbon sugar alcohol with a similar sweetness to sucrose but with 40% less caloric content. Xylitol is mainly used in the pharmaceutical, cosmetic, dental, and food industry (Mohamad et al., 2015). In the food industry, the importance and high demand are mainly due to its low caloric content, low glycemic index and its lack of interfere with the nutritional value of food (Elamin et al., 2012).
At an industrial level, xylitol is mainly produced by the catalytic hydrogenation of birch wood (Prakasham et al., 2009). The process is based on the reduction of D-xyloses to xylitol using dilute acids, high temperatures, metal catalysts, high pressure, and multiple purification steps (Sousa-Aguiar et al., 2014). The hard operation conditions of the process have caused an increase in xylitol price, about 10 times higher than sucrose or sorbitol, which has made this method not profitable (Ur-Rehman et al., 2015). Due to these problems, alternative routes to obtain xylitol are being explored. One of the most promising is the biotechnological route, which use microorganisms capable of converting D -xyloses from hemicelluloses into xylitol. The Candida genus yeasts (C. boidinii, C. tropicalis, C. guilliermondii, and C. shehatae) are the most used to produce xylitol (Cristobal-Sarramian and Atzmüller, 2018; Ur-Rehman et al., 2015).
Agricultural residues are feedstock with great potential to produce xylitol due to their high xylans content present in the form of hemicelluloses (Ur-Rehman et al., 2015). Several studies have explored the biotechnological production of xylitol from many agricultural wastes such as rice husk (Hickert et al., 2013), soybean hull (Cortivo et al., 2018), corn cobs (Wei et al., 2010), sugar cane bagasse (Vaz de Arruda et al., 2017), sorghum bagasse (Ledezma-Orozco et al., 2018), and rapeseed straw hemicellulosic hydrolysate (López-Linares et al., 2018) obtaining interesting data.
In this work, we present an analysis of recent and important investigations related to the production of xylitol by biotechnological ways, covering the main agricultural residues used as feedstock, the main microorganisms used for this purpose, and the possible applications of xylitol in the food industry. Additionally, we review some advantages and disadvantages of the production of xylitol by biotechnological routes.
Agricultural residues
Agricultural residues are any material that remains in the field after harvest such as mixture of stems, leaves, and pods, commonly called straws (Sun, 2010). The processing of crops seeds also generates large amounts of waste, like cobs and husks. Agricultural waste is an abundant source of organic compounds such as cellulose, hemicellulose, lignin, minerals, lipids, proteins, and pectins (Saini et al., 2015). The integral use of these residues can contribute to reduce the adverse effects in the environment; for example, the pollution generated during the open burning of these residues, and at the same time, their transformation into useful products for some industries would help to reduce the production cost of cosmetics, medicines, and food additives (Sun, 2010) among others.
Chemical composition of agricultural residues
Another name for agricultural residues is lignocellulosic materials, because of their cell walls composition, a network of polysaccharides and cross-linked aromatic polymers. The predominant component in cell walls is cellulose, followed by hemicellulose and finally lignin (Peng and She, 2014); cellulose is covalently bound to the hemicellulose, filling the spaces between polysaccharides (Figure 1). Cellulose is the most abundant organic material in nature and constitutes 30-50 % of agricultural waste. Chemically, cellulose is a linear homopolymer formed by the binding of β-D-glucose monomers through β-1,4-O-glucosidic bonds. Cellulose has a linear structure connected by multiple hydrogen bonds between different glucose chains hydroxyl groups (Lee et al., 2014). Such alignment in its structure produces the formation of a fibrous structure with crystalline zones and amorphous zones.
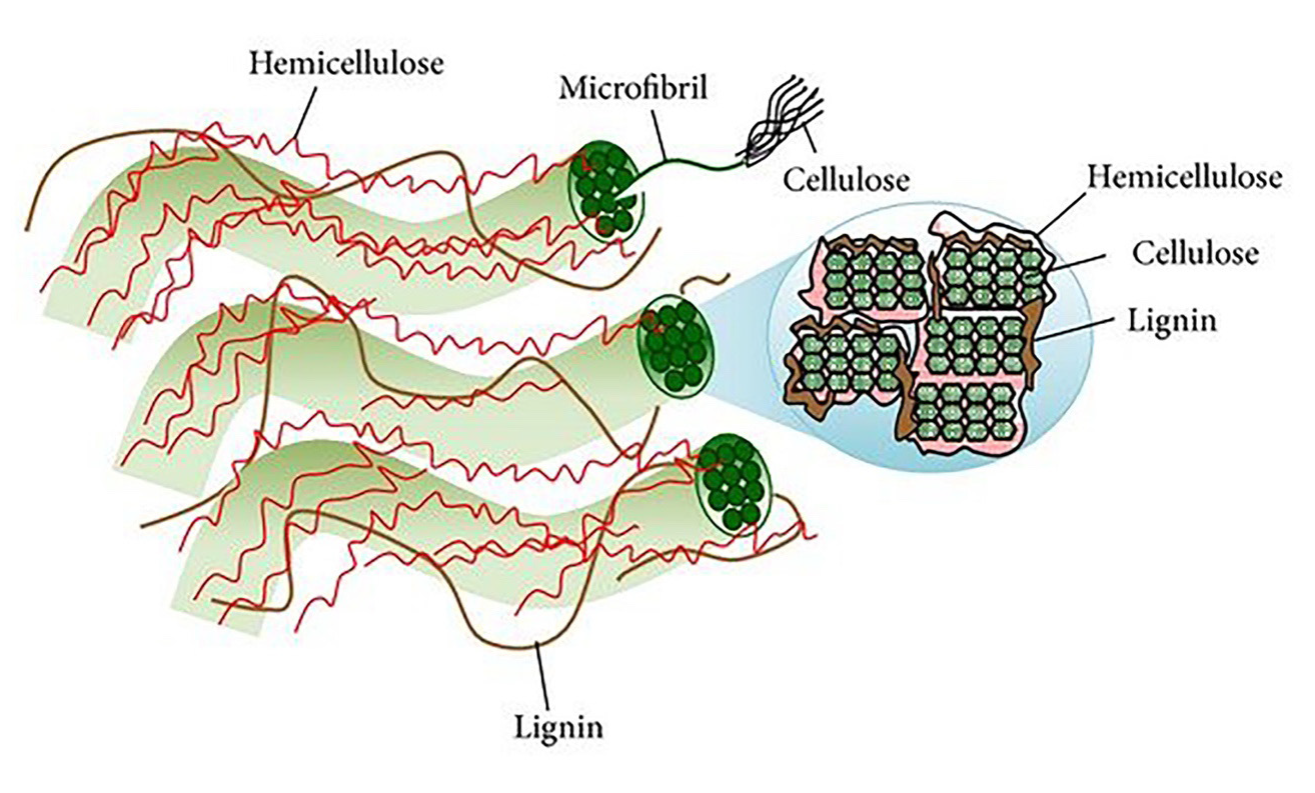
Figura 1 Estructura de la pared celular y sección transversal de las microfibras (cadenas de
moléculas de celulosa incrustadas en un matriz de hemicelulosa y
lignina). Adaptado de Lee et
al., 2014.
Figure 1. Plant cell wall structure and micro fibril
cross-section (strands of cellulose molecules embedded in a matrix
of hemicellulose and lignin). Adapted from Lee et al., 2014.
After cellulose, hemicellulose is the second most abundant polymer in the chemical composition of agricultural waste (25 and 35 %) (Table 1). Unlike cellulose, hemicellulose has a random, amorphous, branched structure with shorter chains composed of several heteropolymers such as xylans, glucomannans, arabinoxylans, galactomannans, and xyloglucans (Isikgor and Becer, 2015). The different heteropolymers that constitute hemicellulose are in turn, made up of 5- and 6-carbon monosaccharides (pentoses, hexoses, acetylated sugars, and uronic acid).
Tabla 1 Composición química de los principales residuos agrícolas.
Table 1. Chemical composition of the main
agricultural wastes.
| Agricultural waste | Cellulose (%) | Hemicelluloses (%) | Lignin (%) | References |
|---|---|---|---|---|
| Rice husk | 31 | 22 | 22 | (Kumar, 2010) |
| Wheat husk | 36 | 18 | 16 | (Bledzki et al., 2010) |
| Barley husk | 34 | 36 | 19 | (Isikgor and Becer 2015) |
| Rice straw | 43 | 34 | 22 | (El-Tayeb et al., 2012) |
| Wheat straw | 40 | 34 | 17 | (Jablonský et al., 2015) |
| Barley straw | 36 | 24 | 6 | (Isikgor and Becer 2015) |
| Oat straw | 31 | 20 | 10 | (Isikgor and Becer 2015) |
| Corn stalks | 35 | 19 | 6.9 | (El-Tayeb et al., 2012) |
| Sugarcane bagasse | 47 | 27 | 21 | (Rocha et al., 2011) |
| Corn cob | 41 | 13 | 35 | (Cortivo et al., 2018; Misra et al., 2013) |
| Peanut shell | 37 | 18 | 28 | (Jaishankar et al., 2014) |
| Almond shell | 32 | 28 | 32 | (Xie et al., 2013) |
Unlike cellulose and hemicellulose, lignin is a nonpolysaccharide amorphous heteropolymer composed of multiple units of phenylpropane, which originate from three aromatic alcohols called monolignols: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol. During the lignification process, the connection of monolignols is through radical coupling reactions to form the lignin polymer. The aromatic constituents of the aromatic alcohols in the lignin polymer are known as p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units. The proportion of these units varies according to the type of plant. The main bonds that make up the lignin polymer are carbon-oxygen and carbon-carbon type (β-O-4, α-O-4, 4-O-5, β-5, β-1, 5-5, β-β). The variability of bonds and monomeric units makes lignin a highly branched polymer, lacking regular order and repeatability. Also, lignin contains various functional groups, including aliphatic and aromatic hydroxyls, carbonyl, carboxyl, and methoxy groups (Buranov and Mazza, 2008).
Biorefineries, Biofuels, And Sub-Products
A biorefinery is a structure capable of producing energy and products of commercial interest through the integral biomass processing (IEA bioenergy Task 42, 2008). The concept of biorefinery comprises a wide range of technologies capable of separating lignocellulosic biomass in its building blocks (carbohydrates, proteins, lipids, and aromatics compounds), where cellulose and lignin are used as precursors to obtain biofuels and chemical products; it is an analogous concept to oil refineries, which produce multiple fuels and petroleum-based products (Cherubini, 2010).
Studies predict that, due to global problems such as climate change and environmental pollution, associated with the increase in use fossil fuels, the constant increase in the prices of fossil resources and their uncertain availability, the viability of oil exploration will decrease soon. This makes the future of some chemical products and food additives, obtained through the chemical conversion of petroleum derivatives, uncertain. For these reasons, the conversion of lignocellulosic wastes into multiple products under the concept of the biorefinery is increasingly explored. Despite the significant advances achieved in this area, their focus is biofuel production (Cherubini, 2010). However, to obtain a significant advance and to get biorefineries commercialized, it is necessary to obtain multiple products besides biofuels. For this, the development of novel and efficient processes to generate valuable byproducts, as well as energy, is essential.
Under the objective of this review, the following sections describe and analyze recent research focused on the biotransformation of agricultural residues in sweeteners, specifically xylitol. Some of these investigations used a biorefinery scheme, taking advantage of the waste generated in the process of converting celluloses to biofuels. Other research focuses on exploring the feasibility of uncommon raw materials (abundant in hemicelluloses) to produce value-added compounds, the use of genetically improved microorganisms to achieve a higher conversion rate, and further research to evaluate the efficiency of fermentation processes to reduce costs and production times. Before reviewing the investigations related to obtaining xylitol using biotechnological routes, we include some essential aspects of the sweeteners, xylitol, and the traditional chemical routes to obtain this sweetener.
Sweeteners
Sweetener is any substance with the ability to impart a sweet taste to foods and beverages (Sharma et al., 2016). The classification can depending on their origin, as natural or artificial, or depending on the caloric content, as high caloric content or low caloric content. There are around 40 different types of sweeteners (Table 2), although the most used in the food and beverage industry are of artificial origin, low caloric content, and high sweetening power. For example, the sweetening power of cyclamate is 24 to 40 times sweeter than sucrose; aspartame, and acesulfame are 100 to 200 times sweeter, while saccharin is 200 to 500 times sweeter. Xylitol has a sweetening power similar to sucrose, but with 40 % lower calorie content (Bellisle and Drewnowski, 2007). Some sweeteners can impart other attributes besides sweet taste, for example, mannitol, maltitol, xylitol, erythritol, and sorbitol add volume or texture to some foods.
Tabla 2 Tipos de edulcorantes.
Table 2. Types of sweeteners.
| Sweeteners | |||||
|---|---|---|---|---|---|
| Nutritive Sweeteners | Non-Sugar Sweeteners | ||||
| Sugars | Natural | Modifie Sugars | Sugar Alcohols | Natural | Artificial |
| Sucrose Dextrose Glucose Fructose Lactose Maltose Galactose Trehalose | Honey Maple syrup Palm sugar Coconut sugar | High-fructose corn syrup Invert sugar | Sorbitol Xylitol Mannitol Erythritol Maltitol Isomaltulose Lactitol | Stevia Thaumatina Pentadin Monelina Brazzein | Aspartame Saccharin Sucralose Acesulfame Cyclamate Neohesperidin |
¿Why sweeteners are being consumed?
Weight loss, dental care, diabetes mellitus, and hypoglycemia are among the reasons for consuming sweeteners or sugar substitutes (Sharma et al., 2016). Sugar substitutes provide a pleasant taste on the palate but contain less caloric content, for this is possible to consume foods and beverages prepared with sugar substitutes without gaining weight (Bellisle and Drewnowski, 2007). Although the use of some artificial sweeteners for this purpose has been questioned (Tandel, 2011; Ur-Rehman et al., 2015), sugar substitutes do not damage the teeth since the microflora of the dental plaque cannot ferment them, consequently, they do not promote the appearance of dental caries (Janakiram et al., 2017; Nayak et al., 2014). Patients with diseases such as diabetes can eat a varied diet when consuming foods prepared with low-calorie sugar substitutes (Kishore et al., 2012). Finally, in patients with reactive hypoglycemia, eating a diet that includes foods that contain sweeteners instead of sugar can control insulin levels produced by the rapid absorption of glucose from the blood stream (Islam, 2011; Islam and Indrajit, 2012).
Today, people pay more attention to the calories they eat and ingredients in the food they ingest. Besides, they demand natural and ecologically friendly products that contain fewer calories, but without sacrificing attributes such as taste or texture of food. This has led to the investigation of new routes to obtain safer sweeteners for consumers, maintaining or even improving the characteristics of artificial sweeteners. An example of this is the biotechnological production of xylitol. Currently, xylitol is the only sweetener produced through the transformation of agricultural waste through biotechnological routes.
Xylitol
Xylitol (C5H12O5) is a five-carbon sugar alcohol with a similar sweetness relative to sucrose, but with lower caloric content. Xylitol contains 2.4 calories per gram, 40 % fewer calories than sugar (Zhang et al., 2014). Naturally, xylitol is present in fruits, vegetables, and some fungi, although in such small amounts that it could not be used for commercial purposes (Ping et al., 2013). On a large scale, xylitol is produced by the catalytic hydrogenation of D-xylose (Prakasham et al., 2009). Xylitol is mainly used in the pharmaceutical, nutraceutical, dental, and food industries (Mohamad et al., 2015). Its importance and high demand in food industry lies mainly in its low caloric content, low glycemic index and it does not interfere with the nutritional value of foods (Elamin et al., 2012). Due to these attributes, the food market demands more and more xylitol production every year. Studies estimate that the demand for xylitol will increase from 190 million metric tons in 2016 to 250 million metric tons for the year 2022 (http://industry-experts.com/verticals/food-and-beverage/xylitol-a -global-market-overview). The growth of the alternative market for sweeteners and the increase in the search for low-calorie sweeteners are two critical factors that have contributed to the increase in demand for xylitol (Dasgupta et al., 2017).
Production of xylitol by chemical routes
The industrial production of xylitol involves the chemical conversion of xyloses derived from hemicellulose hydrolysates rich in xylans from wood residues. In the chemical conversion process, highly pure xyloses are converted to xylitol by hydrogenation, in the presence of a metal catalyst (Ni, Ru, Rh), followed by several purification steps to remove toxic compounds (Figure 2). At the end of the process, xylitol is concentrated and recovered by crystallization, with a purity higher than 98% (Delgado Arcaño et al., 2018).
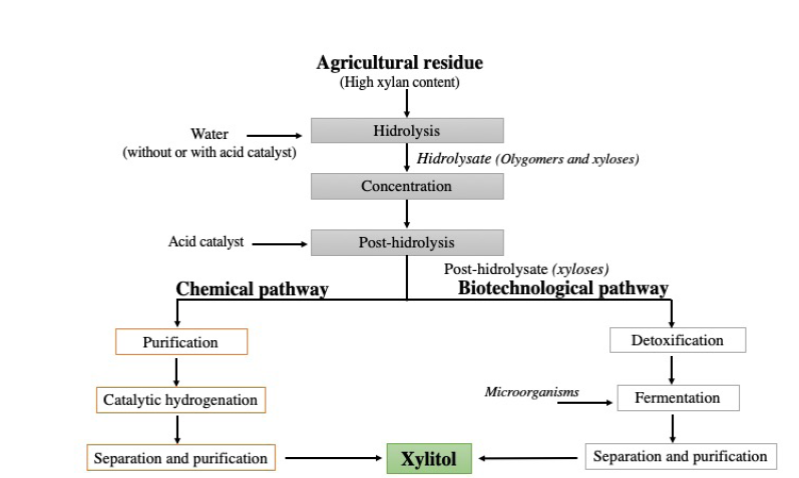
Figura 2 Producción de xilitol por ruta química y biotecnológica (Adaptado de
Vallejos y Area, 2017).
Figure 2. Scheme of xylitol production by chemical or
biotechnological pathways (Adapted from Vallejos y Area, 2017).
The severe conditions of the chemical production process of xylitol (80-140 °C, 31-40 atm, 3-5 hours of reaction), the rigorous purification processes, the high energy demand and the moderate xylitol yield (between 50 and 60 % of xylitol with respect to total xylose) are leading to the search for new alternatives for its production. The use of biotechnological routes to obtain xylitol using lignocellulosic biomass has been reported as an interesting alternative (Vallejos and Area, 2017).
Production of xylitol for biotechnology routes
The basis for biotechnological production of xylitol from lignocellulosic materials resides on xyloses hydrogenation to form hemicelluloses, using several microorganisms such as yeast, bacteria, and fungi, of which, yeasts are best to produce xylitol (Figure 3). Yeasts of the genus Candida (C. boidinii, C. tropicalis, C. guilliermondii and C. shehatae) (López-Linares et al., 2018), are the most used for the production of xylitol; in addition, the yeasts Pachysolen tannophilus, Hansenula polymorpha, Debaryomyces hansenii (Ledezma-Orozco et al., 2018), and Pichia guilliermondii have also been used for this purpose, although to a lesser extent (Table 3). The preference for the genus Candida is due to the high yield of xylitol production, and for being efficient even under limited oxygen conditions. These microorganisms can produce xylitol as an intermediate metabolite of xylulose. For example, Candida guilliermondii, one of the most commonly used yeasts to obtain xylitol has two key enzymes in xylitol metabolism: (1) NADPH-dependent xylose reductase and (2) NADP+ dependent xylose dehydrogenase. The former reduces xylose to xylitol, and the latter oxidizes xylitol to xylulose, and both are induced by xylose (Silva et al., 2004).
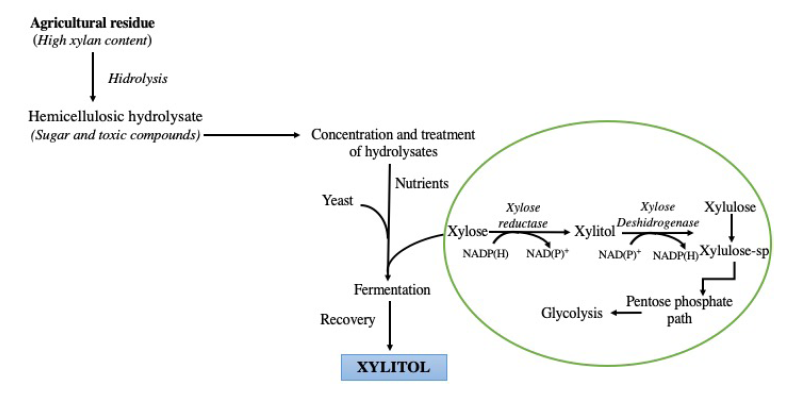
Figura 3 Producción biotecnológica de xilitol (Silva et
al. 2004).
Figure 3. Biotechnological production of xylitol (Silva et al.
2004).
Tabla 3 Producción de xilitol por diferentes levaduras y condiciones de fermentación
utilizando residuos lignocelulósicos como materias primas.
Table 3. Xylitol production by different yeasts and
fermentation conditions using lignocellulosic waste as
feedstock.
| Microorganisms | Hydrolyzed feedstock | Fermentation conditions | Conversion yield of xylose to xilitol (g g−1) | References |
|---|---|---|---|---|
| C. shehatae HM 52.2 | Rice husk | Reaction time 228 h Agitation 180 rpm Temperature 30 °C Aeration speed 0.33 vvm | 0.11 | (Hickert et al., 2013) |
| C. tropicalis CCTCC M2012462 | Corn cobs | Reaction time 100 h, Agitation 200 rpm Temperature 35°C, Aeration speed 0.4 Vvm | 0.71 | (Ping et al., 2013) |
| C. tropicalis | Corn cobs | Reaction time 66 h, Agitation 200 rpm, Temperature 30°C, pH 4.5 | 0.58 | (Misra et al., 2013) |
| C. tropicalis CICC1779 | Corn cobs | Reaction time 24 h, Agitation 210 rpm, pH 6 | 0.77 | (Jia et al., 2016) |
| C. guilliermondii FTI 20037 | Sugarcane bagasse | Reaction time 144 h, Agitation 450 rpm, Temperature 30°C, Aeration speed 0.7 vvm | 0.69 | (Vaz de Arruda et al., 2017) |
| W. anomalus WA-HF5.5 | Rice and soja husk | Reaction time 72 h, Agitation 180 rpm, Temperature 30°C, Aeration speed 0.33 vvm | 0.86 | (Sehnem et al., 2017) |
| Debaryomyces hansenii and C. guilliermondii | Canola straw | Reaction time 72 h, Agitation 200 rpm Temperature 30°C | 0.45 - 0.55 | (López-Linares et al., 2018) |
| C. guilliermondii BL 13 | Soja husk | Reaction time 72 h, Agitation 180 rpm Temperature 23-33°C | 0.46 | (Cunha-Pereira et al., 2017) |
| S. cerevisiae YRH 396 and S. cerevisiae YRH 400 | Soja husk | Agitation 180-300 rpm, Temperature 28°C, Aeration speed 1 vvm, pH 5.5 | 0.45 | (Cortivo et al., 2018) |
Research into xylitol production intensified not only because of the peculiarities of its flavor, but also for the possibility that fermentative processes becomes a viable alternative to current chemical routes. However, the toxic compounds obtained in the hydrolysates of agricultural residues have hampered the production of xylitol through fermentative processes of hydrolysates rich in xyloses. The most reported substrates for the microbial production of xylitol are acid hydrolysates of corn cob (Ping et al., 2013), sugar cane bagasse (Unrean and Ketsub, 2018), oat husk (Cortivo et al., 2018), rice and soybean husk (Cunha-Pereira et al., 2017; Sehnem et al., 2017) (Table 3).
These hydrolysates produce small amounts of toxic compounds such as furfural, acetic acid, and hydroxymethylfurfural (HMF), which can inhibit fermentation and adversely affect the yield of xylitol production. Acetic acid, for example, is a potent inhibitor of yeasts metabolism that convert xylose into xylitol and its effect depends on the concentration and fermentation time. To mitigate this problem, a frequent strategy prior to the hydrolysates fermentation is the use of detoxification processes using, for example, activated carbon (Delgado Arcaño et al., 2018), saturation with lime (Mohagheghi et al., 2006) or ion exchange resins (Kumar et al., 2018). However, detoxification usually reduces the efficiency of fermentation and requires additional facilities that increase xylitol production costs (Fehér et al., 2018). The most feasible way to remove impurities and obtain xylitol while maintaining the biotechnological approach is the use of microorganisms that tolerate inhibitor compounds. These microorganisms can produce xylitol in adequate quantities in the presence of inhibitory compounds (Ledezma-Orozco et al., 2018), high osmotic pressure, limited oxygen conditions even in combination with yeasts such as S. cerevisiae (Ping et al., 2013). For example, Ledezma-Orozco et al. (2018) demonstrated that D. hansenii metabolizes xylose in the presence of acetic acid and furfural.
On the other hand, the combination of yeasts, such as S. cerevisiae and C. tropicalis, are used for the production of cellulosic ethanol (Sehnem et al., 2017). Here, S. cerevisiae can ferment hexoses and C. tropicalis xyloses in a single step, obtaining ethanol from cellulose and xylitol from xyloses (Cheng et al., 2014; Huang et al., 2011; Mateo et al., 2015).
In addition to the use of microorganisms tolerant to inhibitory compounds, there are reports on the use of ultrasonic waves that can improve the production of xylitol. Short intervals of ultrasonic waves applied during the fermentation of sugarcane bagasse hydrolysates can produce an increase of 17 to 20 % in the final yield of xylitol. This increase was attributed to the fact that sonication promotes the uptake of xyloses, reduces the inhibitory effects of the substrate, improves the permeability of the cell membrane causing a rapid diffusion of nutrients from the substrate, which improves fermentation kinetics (Tizazu et al., 2018a).
Recent work resumed the use of immobilized yeasts to produce xylitol, in combination with ultrasound. Tizazu et al. (2018b) reported the use of ultrasound to improve xylitol production from sugarcane bagasse using C. tropicalis MCC 184 immobilized in polyurethane foam. The results of their studies showed that the application of sonication and immobilized yeasts could double the yield of xylitol, and reduce the fermentation time (Tizazu et al., 2018b). The advantages of the use of immobilized yeasts compared to the use of suspended yeasts are higher cell density within the bioreactor, greater productivity and stability, reuse of the yeasts, easy separation of the yeasts from the substrate and reduction of toxic products at the end of the process (Wang et al., 2012).
Several scientific research reviewed the molecular strategies, challenges, progress and perspectives to improve biotechnological production of xylitol using lignocellulosic residues (Dasgupta et al., 2017; Delgado Arcaño et al., 2018; Naidu et al., 2018; Pal et al., 2016; Venkateswar Rao et al., 2016). All of these investigations provide a clear idea of the advantages and disadvantages of xylitol production using biotechnological routes (Table 4). These investigations reported that more research is necessary related to the economic viability of the production of xylitol from lignocellulosic waste, the recovery and separation of xylitol from the fermentation media, the crystallization processes of xylitol, as well as parameters for scale the production of xylitol.
Tabla 4 Ventajas y desventajas de la producción de xilitol mediante rutas biotecnológicas.
| Xylitol production through biotechnological pathways | |
|---|---|
| Advantages | Disadvantages |
| Use of renewable raw materials Use of multiple microorganisms Eco-friendly processes Moderate production conditions Less generation of toxic effluents Lower price of xylitol Non-caloric sweetener | Difficult recovery Multiple steps of purification Relatively long production times High production cost Difficult to scale at industrial level |
Potential applications of xylitol in the food industry
Current uses of xylitol includes sweetener in jams, jellies, desserts, confectionery, chewing gum, and baked goods. The most important use has been as a substitute for sugar in confectionery products and baked goods (Ur-Rehman et al., 2015). In confectionery products such as candy or chewing gum, the use of xylitol is important because it provides a quick source of sweetness, flavor, and a refreshing effect. In general, xylitol is used exclusively or in combination with other sugar substitutes of sugar-free chocolate, hard candies, and water fillings (Ur-Rehman et al., 2015).
In baked products, xylitol reduces the caramelization of sugars, which produce a darkening of the product due to the Millard reactions that occur between sugars and proteins. These reactions do not occur by the addition of xylitol, since it does not contain aldehyde or ketone groups. Investigations on the potential application of xylitol include baked goods such as bread and biscuits. It has been shown that biscuits prepared by replacing sucrose with xylitol up to 50% are sensory acceptable, microbiologically safe and has a longer shelf life (Mushtaq et al., 2010; Winkelhausen et al., 2007).
The replacement of sucrose by xylitol (obtained from the biotechnological processing of banana peels) has been reported in the preparation of rusks (Rehman et al., 2013). The addition of more than 50% of xylitol in this type of bread decreased color and increased hardness of the product (Muhammad et al., 2012). The addition of xylitol affects the rheological properties of the dough; mainly, the addition of high percentages of xylitol produced a discontinuous matrix of gluten, which do not entirely covers the starch granules. Consequently, this affects the sensory quality of bread (Sun et al., 2014). There are reports indicating that the optimum amount of xylitol to impart positive sensory attributes in baked wheat bread (volume, hardness, texture, crumb color, and flavor) is between 5% and 10%. Outside this range, xylitol deteriorates dough properties and consequently of the bread (Sun et al., 2014). In addition, xylitol has great potential as humectant ingredient in foods because it is highly hygroscopic in nature, absorbs water in food (Mushtaq et al., 2010), and it has low glass transition temperature Tg (20oC lower than sorbitol) (Young and O’Sullivan, 2011).
In general, although there is little information regarding the use of xylitol in bakery products, research shows that xylitol can be used to replace sugars in different products such as cookies, bread, rusk, and confectionary products without affecting their physicochemical characteristics and shelf stability.
Conclusions
The production of xylitol is growing continuously due to the high demand for the manufacture of products for oral hygiene, pharmaceuticals, cosmetics and food sweeteners (baked goods, jams, gelatins, chewing gum, ice cream, etc.). Besides, the consumption of xylitol has shown positive effects in the prevention or treatment of diseases such as diabetes and obesity. The production of xylitol using various agricultural residues and alternative routes to chemical routes is widely investigated. The development of biotechnological processes using improved microorganisms (yeast, fungi, bacteria, and microbial consortia), the use of ultrasonic waves, systems with immobilized microorganisms, among other strategies are helping to obtain xylitol and xylitolbased products safer for consumers, although for now with a price above those obtained by chemical synthesis. The xylitol cost production by biotechnological routes on an industrial scale depend on the technologies used to obtain and purify xylose, convert xylose to xylitol and recover/purify xylitol.
Research on the use of low-cost substrates, the development of multipurpose microorganisms capable of tolerating extreme working conditions and the regulation of processes may make it possible to produce xylitol economically feasible.











 nueva página del texto (beta)
nueva página del texto (beta)


