Introduction
Permanent junctional reciprocating tachycardia (PJRT) is an infrequent form of sustained orthodromic supraventricular tachycardia (SVT) in children; clinical and electrocardiographically is incessant and generally refractory to antiarrhythmic drugs (AAD). It was described by the French electrophysiologist Philippe Coumel1 in 1967; it is characterized by a reciprocal rhythm that involves a macroreentry circuit with anterograde conduction over the AV (auriculoventricular) node-His-Purkinje axis and a concealed accessory pathway with decremental conduction properties as the retrograde limb. This report aimed to describe the clinical course and results of definitive treatment with catheter ablation in children with Coumel tachycardia.
Clinical cases
This retrospective study was conducted at a single tertiary heart center and included five consecutive pediatric patients, mean age 11 ± 3 years (range 6 to 14, median 12) who were referred to the arrhythmia clinic for electrophysiological study and definitive treatment with catheter ablation between August 1996 and August 2010. Indications for interventional treatment were symptomatic persistent tachycardia and AAD refractory. All parents supplied written informed consent for interventional electrophysiological study and catheter ablation.
Electrophysiological study
Under local anesthesia and conscious sedation, a 6-Fr quadripolar Josephson-curve catheters were introduced percutaneously via right and left femoral veins and positioned under fluoroscopic guidance in the high right atrium-right ventricular apex and His-bundle region. A 6-Fr decapolar steerable catheter was introduced via the internal jugular vein and placed into the coronary sinus with the proximal electrodes positioned close to the ostium under local intracardiac electrograms guidance. Bipolar intracardiac electrograms were filtered between 30 to 500 Hz and displayed at a sweep speed of 100 to 200 mm/s. Programmed stimulation was performed from the right ventricular apex, right atrium, and coronary sinus.
Catheter ablation procedure
The ablation catheter, a 7-Fr steerable quadripolar catheter, 4 mm distal-tip electrode (Mansfield/Webster or RF Marinr MC USA), was advanced via the right femoral vein for precise mapping along the posterior aspect of the atrial septum and the coronary sinus ostium under 30° right and 45° left anterior oblique fluoroscopic views. The radiofrequency (RF) current was delivered between the distal-tip electrode and a dispersive pad applied to the left subscapular region selecting a power of 30 W and a maximum temperature of 70°C. If successful pulses, RF was applied for 120 s otherwise, it was interrupted at 10 s. After successful pulses, reinduction of SVT was tried by programmed stimulation.
Follow-up
The follow-up was conducted with the arrhythmia clinic and congenital heart disease services. All patients were observed overnight in the pediatric postoperative intermediate intensive care room. Then, in the pediatric cardiology area for clinical observation, ECG (electrocardiogram) and echocardiographic monitoring to detect possible complications before discharge. The patients were discharged in the following 24 hours with aspirin 100 mg/day for 2 months. The follow-up was done in the outpatient arrhythmia clinic every 3 months, at least up to one-year post-ablation. Recurrence was defined as the return of clinical symptoms and ECG documented Coumel tachycardia.
Results
During 14 years, three males and two females (five patients, 0.35 cases per year) were evaluated for SVT, the preexcitation variant of Coumel tachycardia. The first episode of SVT appeared at a mean age of 10.4 ± 4.8 years (range 2 to 14, median 12) with a mean evolution of 7.4 ± 9.4 months (range 1 to 24, median 4).
Clinical characteristics
Symptoms were present in all patients with a predominance of palpitations and dyspnea (100%), and coexisting dizziness (20%). Four of five patients had normal structural heart; however, a 6 year-old female showed symptoms and signs of congestive heart failure (CHF) with an echocardiogram showing a dilated cardiomyopathy pattern (generalized hypokinesia with left ventricular ejection fraction 0.38). No patient showed syncope or was resuscitated.
Antiarrhythmic treatment and outcome
In the setting of incessant Coumel tachycardia, acute treatment in the emergency service was always unsuccessful with autonomic vagal maneuvers or intravenous (IV) verapamil, adenosine or amiodarone. AAD therapy temporarily stopped the tachycardia with almost immediate spontaneous onset. Electrical cardioversion was not indicated due to the same expected effect as with AAD treatment. The patients were initially admitted to the intermediate critical care or pediatric unit and treated with a combined scheme of AAD, then followed-up in an outpatient basis. The response was unsuccessful in acute and at mid-term outcome in all that included combination of oral propafenone plus propranolol, digoxin plus propranolol, quinidine plus propranolol or IV amiodarone load followed by maintenance oral dose (only a relative decrease in the heart rate was obtained in all).
ECG characteristics
In all cases, SVT was documented by resting 12-lead ECG at the time of the first evaluation in the pediatric or cardiac emergency service and by 24 hours-ECG Holter monitoring during the follow-up. All patients had the nearly incessant form of SVT, stopped only by a few sinus beats and self-onset recurrent ECG pattern (Figure 1).

Figure 1 12-lead ECG recording during Coumel tachycardia. Narrow QRS tachycardia (145 bpm) with characteristic long R-P' interval (RP' > P'R) and characteristic retrograde negative P' waves in II, III and aVF (arrows) as well as in lateral precordial leads.ECG: electrocardiogram.
Diagnosis of Coumel tachycardia was based on the classical surface ECG criteria: presence of incessant narrow QRS tachycardia with characteristic retrograde negative P' waves in II, III and augmented vector foot (aVF) leads, and isobiphasic or negatives in septal and left lateral precordial leads, a RP' interval longer than the P'R interval (R'P > P'R), and 1:1 atrioventricular conduction (Figure 2). During the brief sinus rhythm (SR) beats, the PR interval was normal without delta wave. The characteristic ECG pattern, long R-P' was consistent with the slow retrograde ventriculo-atrial conduction but not immediately diagnostic of Coumel tachycardia, because atypical (fast-slow) AV nodal reentrant tachycardia and low right septal atrial tachycardia have a similar ECG pattern (RP'> P'R), also are incessant and have earliest atrial activation near the coronary sinus ostium which required a precise differential diagnosis by electrophysiologic study.
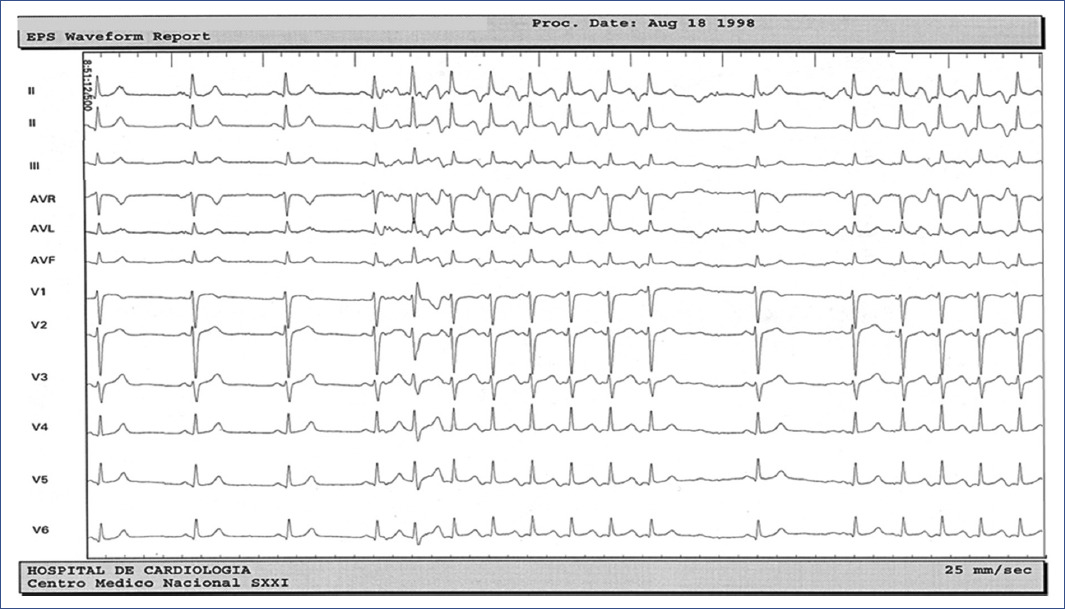
Figure 2 12-lead ECG recording during Coumel tachycardia. Typical presentation of incessant pattern that stops and is followed by a few sinus beats that reinitiates the tachycardia by spontaneously shortening of the PP cycle.ECG: electrocardiogram.
The mean SVT rate was 152 ± 5 beats/min (range 143 to 158, median 154) with a RP' interval 306 ± 24 ms (range 273 to 333, median 313). Based on the 24-hour ECG Holter monitoring, the Coumel tachycardia density per day was > 85% in all patients.
Electrophysiological and catheter ablation characteristics
After spontaneous or by pacing termination, the tachycardia spontaneously resumed with a critical shortening of the PP interval in most cases and also by premature atrial or premature ventricular complexes.
Participation of an accessory AV pathway was confirmed in all cases by a premature ventricular extrastimuli introduced during tachycardia at the time when the His bundle was refractory, resulting in shortening of the atrial cycle length without disturbing the sequence of retrograde atrial activation. Also, it was confirmed decremental ventriculo-atrial conduction during continuous incremental ventricular pacing and premature ventricular extrastimuli or incremental ventricular pacing ended tachycardia without retrograde conduction to the atrium, supporting a reciprocating rhythm2,3; then, excluding atypical AV nodal reentrant tachycardia and low right atrial tachycardia as potentially alternative mechanisms (Figure 3).

Figure 3 Endocardial mapping during tachycardia. The earliest retrograde atrial electrogram (A) is recorded near the coronary sinus ostium (CSos, arrow), basal A-A interval 370 ms. A premature ventricular extrastimuli (S1) introduced at 250 ms when the His bundle is refractory results in shortening of the A-A interval (300 ms) without disturbing the sequence of retrograde atrial activation. The HA' interval during tachycardia is > HA' interval HA' interval during ventricular extrastimuli (310 ms vs 295 ms) and the difference between the post-pacing interval (PPI 460 ms) and the tachycardia cycle length (TCL 370 ms) is 90 ms (All of these criteria confirmed the participation of an accessory AV pathway).CS: coronary sinus electrograms from distal to proximal, H: His bundle electrogram, V: ventricular electrogram.
Endocardial mapping during tachycardia showed an earliest septal retrograde atrial activation recorded at the proximal coronary sinus ostium electrogram. In all cases the accessory AV pathway location was confirmed to be in the right posteroseptal region, then the ablation was attempted from the atrial aspect of the tricuspid annulus adjacent (anterior and slightly below) to the coronary sinus ostium in four cases and ablation was delivery inside the coronary sinus ostium (5 mm) in one case. Ablation targets was with local earlier retrograde atrial activation than reference from de proximal coronary sinus electrogram during ongoing tachycardia, and the successful application site was obtained with a shortest endocardial local A-surface P' interval, mean value of –30 ms, (range –16 to –50, median –33) (Figure 4). Fragmented atrial electrograms were found at the atrial insertion of the accessory pathway in three cases.
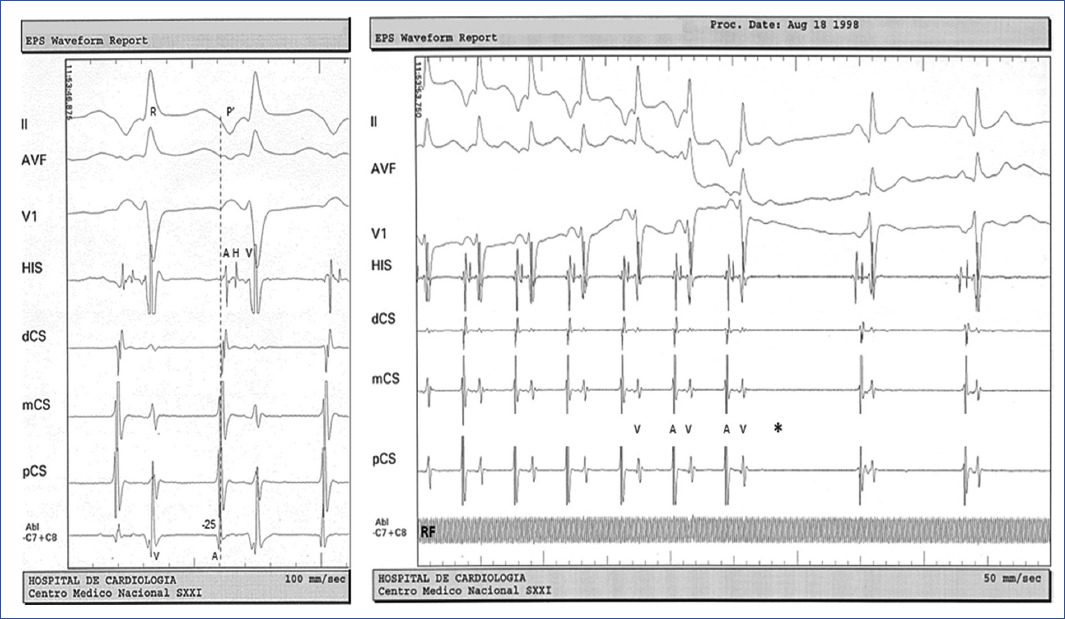
Figure 4 Endocardial mapping and ablation. Left: during sustained tachycardia, the atrial aspect of the tricuspid annulus (right posteroseptal region) is mapped adjacent to the coronary sinus ostium (pCS). Ablation target (Abl) was located earlier than the reference from de proximal coronary sinus (pCS) electrogram with a local atrial (A) electrogram to surface ECG P' interval (dashed line) of –25 ms. Right: at the target site, the application of radiofrequency current (RF) interrupts the tachycardia at the level of the retrograde limb of the circuit (electrogram V not followed by electrogram A, asterisk).ECG: electrocardiogram.
Successful radiofrequency (RF) catheter ablation of the anomalous accessory AV pathway, the anatomical substrate of Coumel tachycardia was obtained with a single session in each case. A mean of 5 ± 3 applications (range 1 to 8, median 3) of RF current per procedure were applied, with a mean power of 28 ± 2 Watts (range 26 to 30, median 28) with mean temperature of 61°C ± 3°C (range 59 to 67, median 63); 20 W/60°C for 30 s inside the coronary sinus ostium in one patient. Total fluoroscopy exposure was 17 ± 9 min (range 5 to 26, median 20). No complications occurred during RF catheter ablation procedure. At a mean follow-up of 24 ± 16 months, all patients were asymptomatic and recurrence-free without AAD treatment. Total recovery of ventricular function (left ventricular ejection fraction (LVEF) 0.65), published in another case series, was documented in the 6-year-old female with tachycardia-induced cardiomyopathy4.
Discussion
Atrioventricular reentrant tachycardia (AVRT) mediated by an anomalous accessory pathway account for 90% of pediatric forms of SVT5. Coumel tachycardia (also-called PJRT) is a preexcitation variant, then a subtype of AVRT and is an infrequent form of refractory and persistent SVT. Generally, occurs in children and infants and accounts for 1% of SVT in this age group5.
It has also been described rarely in the fetal and neonatal period6,7. Coumel tachycardia is usually incessant from birth or infancy, although it may not be recognized until adolescence. Age of clinical presentation is from 1 to 15 years in approximately 40% of cases8,9.
The incessant clinical presentation and usual refractoriness to AAD treatment expose the patient if not early recognized to high risk for developing tachycardia-induced cardiomyopathy as in the case of one of our series that clinically had tachycardia from age 2, but was referred up to 6 years of age with symptoms of severe congestive heart failure (CHF). The remaining patients were diagnosed on time and referred for initial AAD treatment, which only achieved a relative control of the heart rate but without restoring a stable SR, keeping the patients symptomatic. Reports on the AAD therapeutic efficacy of Coumel tachycardia are variable.
In a multicentre study of six tertiary centres, 25 of 32 patients received primary AAD treatment (mean age 15 years). AAD therapy was considered effective in terms of conversion into stable SR in 24% and partially effective in 32%. During follow-up (mean 10 years), drug therapy was discontinued in only 20% of patients8.
Another retrospective multicentre study of seven pediatric institutions included 85 patients over a period of 32 years with median follow-up of 8.2 years. Almost every patient (97%) received initially AAD treatment reporting successful in 84% but 79% received at least two to nine consecutive AAD. Drug-related side effects occurred in 14% but two patients died suddenly for persistent CHF one month and five years after diagnosis9.
From our point of view, these results must be taken cautiously in terms of what they considered complete success: conversion to stable permanent SR or predominant SR alternating with non-sustained tachycardia (< 30 s, low heart rate: < 110 beats/min) or partial success: heart rate reduction > 30% associated with periods of sustained SR on 24-hours Holter monitoring; perhaps valid definitions for that time, but not necessarily at present. Therefore, the results were based principally on the electrocardiographic response and not strictly on symptom improvement, because there is no significant difference between symptomatic and asymptomatic patients in terms of relative hear rate control or tachycardia rate with increased risk of impaired left ventricular function. This is because more than the ventricular rate, is the tachycardia density per day that seems to be the main discriminating variable contributing to left ventricular impairment and subsequent clinical signs of CHF. In our series the tachycardia density was > 85% on 24-hours Holter monitoring.
Spontaneous or intermittent disappearance of Coumel tachycardia has also been reported from 2 to 22%8-10. These data demonstrate a wide clinical spectrum of Coumel tachycardia with appearance most often in early childhood, and patients may present with clinical or echocardiographic findings of impaired ventricular function compatible with tachycardia-induced cardiomyopathy.
Symptoms of CHF are more common in younger patients8. In the Vaksmann et al.9 series, 67% of patients presented before 1 year of age and three of them with intrauterine tachycardia had hydrops fetalis. At the time of referral, 28% presented with varying degrees of CHF, and was more common in infants than in children older than 1 year9. In the Dorostkar et al. series, left ventricular performance was impaired in 11 of 21 patients (52%) with clinical symptoms of severe CHF in infants (36%). The ventricular function was reversible after successful catheter ablation.10
Coumel tachycardia can be difficult to treat. As it is usually refractory to AAD management, it requires treatment with more than a single antiarrhythmic agent. Furthermore, there are no ideal or even satisfactory antiarrhythmic medications for this tachyarrhythmia10.
The effectiveness of catheter ablation procedure has been demonstrated for the definitive treatment of Coumel tachycardia in all the pediatric age groups (including infants and early childhood)9,10. In 1995, Zalstein et al.11 reported the first case of successful treatment with RF ablation in a 3-month-old infant with incessant SVT from 4 weeks of life with tachycardia-induced cardiomyopathy.11 Barbero et al.12 included 16 patients: nine cases underwent to RF catheter ablation during the neonatal period (median age 20 days, median weight of 3.4 kg); the remaining patients underwent catheter ablation at a median of 8 months of age12. In all group a simplified single catheter ablation technique was used achieving success in 93% (1.1 sessions) without major complications. After a median follow-up of 10.4 years all patients have a good quality of life. In a retrospective review of 110 infants (57%; aged < 1 year) managed at eleven institutions during a period of 11 years with tachycardia-induced cardiomyopathy observed in 18%; the catheter ablation procedures had a success rate of 90% without major complications13.
Similarly, the catheter ablation procedure in children has been reported with high success rates9,10. Nine patients were treated with RF catheter ablation, after 6.5 ± 4.4 years of medical treatment, at a mean age of 12.5 ± 1.3 years and successful RF ablation (1.2 sessions) was obtained in all patients14. A recent publication comprised 22 pediatric patients diagnosed in a 10-year period in two pediatric electrophysiology institutions. The mean age was 3.13 ± 4.43 years (range 0 to18) with mean weight 18.22 ± 19.68 kg. The overall success with catheter ablation was 95.4% (1.1 sessions) without complications15.
The vast majority of catheter ablations have used RF energy, but also has been proposed the potential clinical utility of cryoenergy. The cryoablation was safe and successfully accomplished in four patients, mean age 14 ± 5 years16.
In infancy and early childhood the primary management is controversial, and some authors have suggested that AAD treatment is the first option and catheter ablation should be reserved for children and adolescents or only in those cases presenting with left ventricular dysfunction including infants8-10,14. However, the experience accumulated in recent years supports that may be indicated even in infants at an early stage specially when AAD treatments are not rapidly effective and ventricular function worsens12. This is supported because the normalization of ventricular function is fully demonstrated and catheter ablation has a high success rate and major complications are rare.
The classic ECG led Coumel et al1. to suggest that the retrograde limb of the tachycardia circuit had properties of slow conduction which explains the long RP' interval.1 The exact anatomic and physiologic nature of this retrograde limb remained unclear. Gallagher et al. considered that the retrograde limb represented an accessory AV node in the posterior septum, while Farré et al.18 suggested that the limb was a posteroseptal anomalous accessory AV pathway with decremental functional properties.17,18 The anatomical-functional substrate of Coumel tachycardia was definitively documented in 1984 by Critelli et al. in a postmortem study that revealed an accessory AV connection joining the ventricular myocardium from the right posterior septum region to the lower rim of the coronary sinus ostium.19 This accessory AV fiber follows an oblique course with pronounced tortuosity bridging the AV sulcus. As a result of changing cross-sectional area, such an accessory AV pathway might exhibit slow conduction, thus explaining its decremental characteristics. The majority of accessory AV pathways in Coumel tachycardia are localized in the right posteroseptal region, near the coronary sinus ostium, but also less frequent in other areas (left posteroseptal, mid and anterior septum, and rarely in the right and left free-wall of the AV annulus)10,12,15,20.
In conclusion, Coumel tachycardia is a rare form but important cause of SVT occurring in infants and children. The circuit is an orthodromic AV reentry with a concealed, slow conducting accessory AV pathway as the retrograde limb of the circuit that characterizes the typical ECG with a long RP' interval (RP' > P'R) with negative P' waves in II, III and aVF and usually in V3 to V6 leads. This tachyarrhythmia is usually refractory to medical management and due to the incessant nature may lead to tachycardia-induced cardiomyopathy. The catheter ablation procedure is an effective and safe therapy and should be used early in the management of children and in infants based on a strictly individual selection basis. Regression of left ventricular dysfunction is the rule after successful catheter ablation.











 nueva página del texto (beta)
nueva página del texto (beta)


