Introduction
Joubert syndrome is a rare genetic condition with a 1:80,000–1:100,000 prevalence1. This syndrome was described in 1968 by Dr. Marie Joubert in a family presenting with respiratory pattern abnormality (hyperventilation), abnormal eye movements, ataxia, intellectual disability, and cerebellar vermis agenesis2.
Joubert syndrome has often been described with an autosomal recessive inheritance pattern, although heterozygous variants in the ZNF423 and OFD1 genes indicate dominant and X-linked inheritance, respectively3. It is classified within the group of diseases known as ciliopathies4. The hallmark of this syndrome is the malformation of the brain and cerebellum known as the "molar tooth sign," hypotonia, and neurodevelopmental delay5.
This disease manifests initially in the neonatal period with alterations of the respiratory pattern (tachypnea or neonatal episodic apnea). Subsequently, this pattern improves, and new neurological symptoms appear, such as hypotonia or delayed psychomotor development. It has a clinically heterogeneous picture, which may be associated with renal, hepatic, or ocular alterations. Periodic complementary tests should be performed as part of the active search for these alterations6.
Clinical case
We describe the case of a male newborn whose mother was 25 years old, second gestation with adequate prenatal control. Two structural ultrasounds were performed, which reported the measurement of cisterna magna in the 95th percentile. It was decided to terminate the pregnancy by the abdominal route at week 37 due to cholestasis associated with gestation.
The newborn with an irregular respiratory effort required a cycle of positive pressure ventilation: APGAR at 1 min was 8 and 9 at 5 min. At the nursery, the patient presented persistent tachypnea, so an oxygen hood was placed. At 6 h of life, he was admitted to intermediate therapy with high-flow nasal prongs and a diagnosis of transient tachypnea of the newborn. Capillary blood gas showed compensated respiratory acidosis with normoxemia, and a chest X-ray showed lung volume of nine intercostal spaces.
On physical examination, the patient showed decreased muscle tone, weak sucking reflex, hypertelorism, blepharoptosis of the right eye, a flat nasal bridge with a short nose, and postaxial polydactyly of the left hand. He presented no other pathologic data.
The patient was on fasting during the first 48 h of life due to persistent tachypnea > 80/min. When the enteral feeding was restarted, he presented stridor and increased respiratory rate > 100/min. In addition to tachypnea, he presented abundant secretions since birth and polydactyly. An esophagogram was performed, which showed no evidence of a tracheoesophageal fistula, but an alteration in the swallowing mechanics shown by the passage of contrast medium secondary to intermittent dysfunction in the closure of the larynx.
Given the persistence of bradycardia, an electrocardiogram showed sinus bradycardia, and an echocardiogram reported a structurally healthy heart. In addition to the bradycardia, episodes of apneas were identified, so caffeine management was initiated.
The combination of clinical data (sinus bradycardia, swallowing mechanics disorder, hypotonia, apneas, and blepharoptosis) lead to an investigation for a central hypotonic syndrome.
A brain magnetic resonance imaging (MRI) showed a spectrum of midbrain and hindbrain malformations characterized by symmetrical thickening and elongation of the upper cerebellar peduncles with a vertical arrangement, giving the midbrain a molar tooth appearence (Figure 1). The cerebellar hemispheres showed reduced volume and alteration (disorganization) in their folial pattern. The vermis was also hypoplastic and dysplastic, mainly toward its superior portion. The cisterna magna was wide (Figure 2). It was concluded as a Joubert's syndrome diagnosis.

Figure 1 Brain MRI, axial plane, T1-weighted. There is a classic aspect of molar tooth of the midbrain secondary to thickening and elongation of the superior cerebellar peduncles.
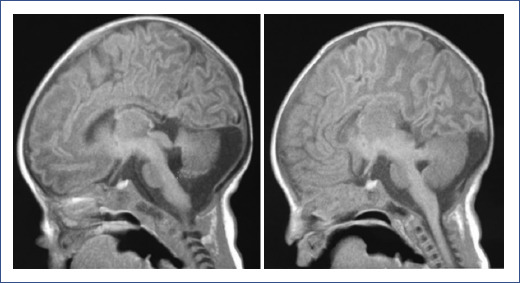
Figure 2 Brain MRI, T1, sagittal and parasagittal planes, showing hypoplasia and dysplastic aspect of the superior cerebellar vermis and cerebral hemispheres, associated with folial disorganization and wide cisterna magna, in addition to thinning of the corpus callosum.
A polysomnography showed apneas of central origin. In addition, the patient presented normal auditory-evoked potentials and visual potentials with delayed response. Electroencephalogram showed no abnormal activity.
As part of the evaluation of Joubert syndrome, we performed a renal ultrasound, in which bilateral nephronophthisis was reported, and a Schwartz glomerular filtration rate of 37.2 mL/min was calculated. Additionally, the hepatic ultrasound showed no alterations. The ophthalmology evaluation reported aponeurotic ptosis of the right eye and normal fundus in both eyes.
A genetic panel analysis based on next-generation sequencing technology (Invitae, Corp.) and deletion/duplication analysis of 102 genes related to ciliopathies was performed. We found a heterozygous variant c.719T>C (p.Ile240Thr) in the CEP104 gene, and a heterozygous variant c.481G>A (p.Asp161Asn) in the TMEM231 gene. Both variants were classified as variants of uncertain significance. Homozygous or compound heterozygous variants in these genes cause Joubert syndrome, and digenic inheritance has been identified at gene loci related to the etiology of Joubert syndrome7.
Discussion
Transient tachypnea of the newborn is the first cause of admission to neonatal care therapy. We present the clinical case of a newborn with initial clinical manifestations and risk factors for this diagnosis (male, early term, and cesarean birth). However, during his stay, the patient had an atypical evolution, showing neurological signs and minor dysmorphias that led us to rule out causes of central origin. The "molar tooth sign," pathognomonic of Joubert's syndrome, was detected on MRI. Once recognized, no other differential diagnosis was established. This radiological finding is caused by the lack of fiber decussation of the superior cerebellar peduncle, leading to the elongation of the peduncles; additionally, the peduncles have a more horizontal course, and there is an enlargement of the interpeduncular cistern. Other primary imaging features of Joubert syndrome are the absence of the cerebellar vermis and deformity of the fourth ventricle8.
Currently, more than 35 genes have been associated with Joubert syndrome8; in our case, alterations in the TMEM231 and CEP104 genes were reported. Although the variants found in this patient have been reported to be of "uncertain significance," variant prediction programs (SIFT, PolyPhen-2, and Align-GVGD) were used. In the case of the c.719T>C (p.Ile240Thr) variant in CEP104, all programs suggested that it could be a disruptive variant. In contrast, in the c.481G>A (p.Asp161Asn) mutation in TMEM231, the prediction algorithms were inconsistent, or the information about structure and function was unavailable.
CEP104 is located on 1p36.31 and encodes the 104kDa centrosomal protein, required for ciliogenesis and for the structural integrity of the cilia endings9. TMEM231 is located on 16q23.1 and encodes the transmembrane protein 231, which is part of a protein complex at the base of the cilium that acts as a barrier to restrict protein diffusion between the plasma and ciliary membranes10.
Considering the patient's phenotype and the importance of CEP104 and TMEM231 in ciliary formation and function, and reports of digenic inheritance, the molecular findings were used for follow-up, prognosis, and genetic counseling. In our case, the variants were not candidates for segregation in the parents due to their uncertain significance. However, a 25% recurrence risk was provided to the parents, informing them of the limitations of the result. The mutations should be reanalyzed, as they may be reclassified as pathogenic in the future.
Although many genes can be affected, the genetic cause can currently be determined in 62% of individuals with Joubert syndrome8. Identifying the underlying genetic defect can help define the prognosis of the disease and guide selective detection of secondary diseases, and allow accurate genetic counseling, the option of prenatal diagnosis, and preimplantation of genetic diagnosis11.
Prenatal diagnosis of Joubert syndrome by ultrasound is rare. Medical sonographers can usually observe non-specific features, such as widened posterior fossa and cerebellar vermis dysplasia. However, these signs can also be found in other malformations, such as Dandy–Walker malformation and pontocerebellar hypoplasia, among others12. Based on obstetric ultrasound findings and complemented with fetal MRI, prenatal diagnosis of Joubert's syndrome is now possible during the third trimester of gestation13. However, in the case of our patient, prenatal diagnosis was not performed since the only abnormal finding reported was the measurement of cisterna magna in the 95th percentile.
An epidemiological study conducted in Italy in 284 patients with Joubert syndrome showed that the mean age at diagnosis was 6.6 years. An early diagnosis is difficult because Joubert syndrome does not have an easily recognizable facial appearance14.
The clinical spectrum of Joubert syndrome may be accompanied by some alterations of other organs, such as retinal defects, nephronophthisis, and hepatic fibrosis; other infrequent conditions include chorioretinal or optic nerve colobomas, congenital cardiac malformations, situs inversus, skeletal dysplasia, and midline defects15.
Eight subtypes of Joubert syndrome have been described: classic, with retinal disease, renal disease, oculorenal disease, liver disease, oral-facial-digital features, acrocallosal features, and asphyxiating thoracic dystrophy features16.
A study of 16 patients with Joubert syndrome showed cerebellar vermis hypoplasia/aplasia and apnea present in all patients, polydactyly was present in three patients, renal problems with cysts were present in five, and 11 had abnormal electroretinograms17. In the case of our patient, he presented all the previously mentioned findings. However, only the fundus has been explored in the ophthalmologic approach, which was normal; until now, an electroretinogram has not been performed.
Once Joubert syndrome has been diagnosed, it should be complemented with renal and hepatic function tests, hepatic and renal ultrasound, and polysomnography to evaluate apneas of central origin. In addition, evaluation by ophthalmology, pediatric cardiology, genetics, pediatric gastroenterology, rehabilitation, and pediatric neurology should be made2. These studies allow to identify the Joubert's syndrome subtype and provide a better follow-up and long-term prognosis.
According to his clinical features, our patient could be classified within the subgroup of Joubert's syndrome with renal disease; however, he has some orofacial-digital features.
Each patient's prognosis is difficult to define since Joubert syndrome has various related conditions and a wide range of intellectual disabilities11. At present, the most important risk factor in our patient is nephronophthisis; therefore, close monitoring by pediatric nephrology should be maintained to preserve renal function as much as possible.
Treatment of the disease is mainly symptomatic and should be multidisciplinary. It usually includes physical therapy programs and adaptation of education to the cognitive and behavioral characteristics of the patient, and treatment of complications or associated symptoms that may appear. The primary care pediatrician's role should be one of integration and coordination of the different referrals18.
Rare genetic diseases can manifest from the neonatal period with non-specific signs that could be confused with common neonatal diseases. In this case, tachypnea is a very frequent sign in the neonatal stage and can be a symptom of multiple origins, including respiratory, hemodynamic, infectious, hematological, and neurological diagnoses. This case should lead us to reflect on the importance of physical examination and clinical judgment to conduct approaches leading to specific diagnoses and, consequently provide a better treatment plan.
Early diagnosis of Joubert syndrome is reflected in better pediatric follow-up, which impacts prognosis and improving the patient's quality of life with multidisciplinary management and providing genetic counseling.











 nueva página del texto (beta)
nueva página del texto (beta)


