Introduction
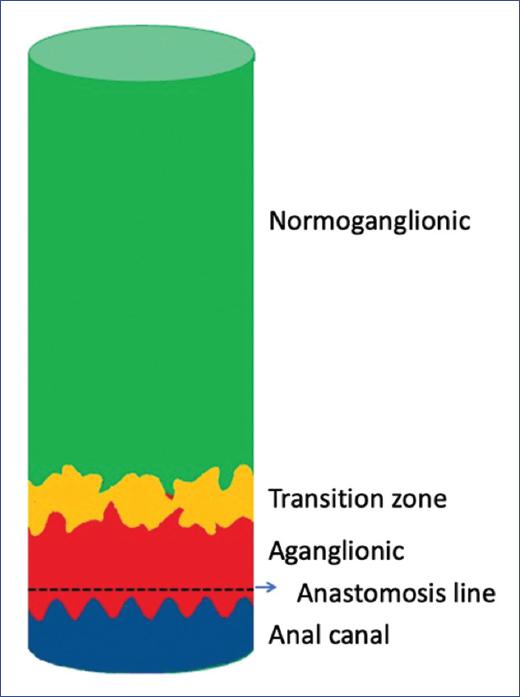
In patients with Hirschsprung´s disease (HD), there is a segment of the bowel called the histologic transition zone (TZ), which is located between the aganglionic and normoganglionic bowel and is ≤ 5 cm in length1 (Fig. 1).
Seven histologic abnormalities have been described in TZ: plexuses with ganglion cells with hypertrophic nerves2, myenteric hypoganglionosis3, hyperganglionosis of the submucosal plexus3, partial circumferential aganglionosis4, ectopia of ganglion cells in the seromuscular layer and lamina propria5, gangliosclerosis1, and fibromuscular dysplasia of the adventitial layer6.
Resection of the entire aganglionic bowel and TZ is one of the goals in the surgical repair of HD. This disease always involves the rectum, and therefore, its resection is part of the treatment. The normoganglionic bowel is then pulled-through and anastomosed to a small segment of the aganglionic rectum measuring approximately 5-10 mm, i.e., the anastomosis is made proximal to the dentate line. The descending bowel forms the "neorectum." The edge of the bowel that anastomoses to the rectum is called the proximal margin. Based on the histology of the proximal margin, there are three types of neorectum. The normoganglionic neorectum (NNR) is composed of a bowel that covers the entire circumference of the proximal margin, has ganglion cells in the submucosal and myenteric plexuses and has no nerve trunk hypertrophy (Fig. 2). The transition zone neorectum (TZNR) has at least one of the following three histologic changes at the proximal margin: partial circumferential aganglionosis, myenteric hypoganglionosis, or hypertrophy of the submucosal nerve plexuses (Fig. 3)1,4,7. Finally, the third type is the aganglionic neorectum (ANR), which has a proximal border without ganglion cells in the myenteric and submucosal plexuses (Fig. 4).
In a pull-through procedure, the surgeon requests a histopathologic study of the proximal margin to ensure the creation of an NNR. Pathologists perform the study of this segment twice and under different conditions. The first time is the intraoperative study, in which frozen tissue, usually stained with hematoxylin and eosin, is evaluated, and the result is reported to the surgeon within minutes during surgery. The second time is known as definitive evaluation and consists of embedding the tissue in paraffin, which provides the pathologist with a higher quality for analysis. In addition, this procedure uses different stains and auxiliary methods, such as immunohistochemistry, to facilitate its evaluation, and the result is reported days after the pull-through. Therefore, the result of the intraoperative evaluation may differ from the final result8,9.
Hirschsprungs associated enterocolitis (HAEC) and postoperative constipation remain a common problem. In recent years, it has been hypothesized that these problems result from a descending bowel in the TZ10 and that reoperation is necessary to resect this zone and perform a redo pull-through11.
The present study aimed to evaluate the presence of HAEC or postoperative constipation and to correlate it with the histopathology of the proximal margin in patients with rectosigmoid HD who underwent a primary pull-through.
Methods
Research methodology
We conducted a retrospective study of all HD patients at Children´s Hospital Colorado (CHCO) from January 2010 to June 2020. Patients with rectosigmoid HD who underwent a pull-through procedure and had clinical follow-up at CHCO during the study period were included. Patients who had surgery or follow-up at another hospital, patients with mechanical obstruction, patients with ANR, syndromic patients, patients with long-segment aganglionosis, and patients with total colonic aganglionosis were excluded from the study. The study was approved by the Colorado Institutional Review Board (IRB #: 20-1891).
Data collection
Patients with HD and a pull-through procedure were identified using the Colorectal Center database and electronic medical records. A review of each patient´s entire record was performed to confirm that they met the inclusion criteria. Demographic and clinical information was then collected: date of birth, sex, race/ethnicity, age at the pull-through procedure, date and type of the procedure. A detailed analysis was performed to determine postoperative outcomes and to identify asymptomatic patients, patients with HAEC events, and constipation.
Operational definitions
Postoperative HAEC was defined when the patient required a program of rectal irrigation and oral or intravenous metronidazole after the pull-through in the setting of low functional bowel obstruction, with smelly diarrhea, fever, explosive bowel movements, distension of the distal colon on a plain abdominal radiograph, or air-fluid levels.
Constipation was defined as decreased, irregular, or absent bowel movements and the need for laxative therapy.
An asymptomatic patient was defined as one who did not require a laxative regimen, had no episodes of HAEC, and had spontaneous bowel movements.
Histopathologic groups
At CHCO, it is a standard practice to send the resected bowel to evaluate the circumference of the proximal margin. Patients intraoperative and final reports were reviewed. NNR was defined when the final report described the presence of ganglion cells in the myenteric and submucosal plexuses and the absence of nerve plexus hypertrophy or other neuroanatomic abnormalities of the transitional zone over the entire circumference of the proximal margin.
TZNR was defined when the final report described at least one of the following three features: (1) ganglion cells in both plexuses with hypertrophy of the submucosal nerve plexuses; (2) absence of ganglion cells in at least one-eighth of the circumference (circumferential partial aganglionosis); (3) myenteric hypoganglionosis.
ANR was defined as the absence of ganglion cells in both plexuses over the entire circumference of the proximal border.
Nerve plexus hypertrophy was defined as submucosal nerve trunks > 40 μm. Nerve diameter was measured with an optical micrometer in all cases. Two pediatric pathologists with expertise in HD independently re-examined the proximal margin if the final report described the presence of ganglion cells but no other neuroanatomic abnormalities of the TZ (i.e., an inconclusive report of the TZ).
Results
Participants
A total of 98 records of patients with HD diagnosis were retrospectively analyzed. Twenty patients were excluded because of long-segment aganglionosis, total colonic aganglionosis, or syndromic HD. In addition, one patient was excluded because the pull-through procedure was performed at another hospital, and six patients were excluded because of anastomotic stenosis; there were no patients with ANR.
A total of 71 patients remained for analysis. Intraoperative diagnosis of the proximal margin revealed ganglion cells in the myenteric and submucosal plexus without nerve trunk hypertrophy over the entire circumference in all 71 patients. The final diagnosis of the proximal margin was normoganglionic in 63 patients and TZ in four patients due to the presence of nerve trunk hypertrophy. The remaining four reports did not describe the presence or absence of hypertrophic nerves or other histopathologic features of TZ. On review by pathologists, two were reported as normoganglionic and two with marked hypertrophy of submucosal nerve plexuses. Therefore, 65 patients had NNR, and six patients had TZNR. None of the six proximal margins diagnosed with TZ showed other neuroanatomical abnormalities described in TZ besides truncal hypertrophy. The concordance between intraoperative and final reports was 91.5%. There were no patients with ANR.
Demographic characteristics
Both groups were predominantly males: 47 (72%) in the NNR group and four (67%) in the TZNR group. The mean age of the 71 patients at the time of primary repair was 11 months. There was no significant difference in mean age between both groups (NNR = 11 months and TZNR = 7 months). Soave-type pull-through was performed in 69 patients, Swenson in one, and Duhamel in one. The median follow-up for the NNR group was 6.80 years (interquartile range [IQR]: 5.82-10.63 years), and for the TZNR group was 5.77 years (IQR: 0.39-10.80 years). All demographic characteristics of both groups are summarized in table 1.
Table 1 Demographic characteristics of groups with NNR and TZNR
| Type of neorectum | |||
|---|---|---|---|
| NNR (n = 65) | TZNR (n = 6) | p-value | |
| Sex | 1.00 | ||
| Female | 18 (28%) | 2 (33%) | |
| Male | 47 (72%) | 4 (67%) | |
| Race | N/A | ||
| African American | 5 (8%) | 0 (0%) | |
| Asian | 3 (5%) | 1 (17%) | |
| Alaskan Indian/Alaskan Native | 1 (1%) | 0 (0%) | |
| White | 40 (62%) | 5 (83%) | |
| More than one race | 1 (1%) | 0 (0%) | |
| Unknown | 13 (20%) | 0 (0%) | |
| Other | 2 (3%) | 0 (0%) | |
| Ethnicity | 1.00 | ||
| Hispanic or Latino | 9 (14%) | 1 (17%) | |
| Not Hispanic or Latino | 53 (82%) | 5 (83%) | |
| Unknown | 3 (4%) | 0 (0%) | |
| Age at surgery (years) | 0.96 (2.51) | 0.61 (0.47) | 0.74 |
NNR: normoganglionic neorectum; TZNR: transition zone neorectum.
Postoperative outcomes
Overall, of the 71 patients, 42 (59.1%) had HAEC, constipation, or both; 38 had NNR, four had TZNR (p = 0.818). Moreover, 29 (40.8%) patients were asymptomatic. All patients with HAEC or constipation, regardless of neorectum type, received medical treatment, and none underwent reoperation.
Of the 65 patients with NNR, nine had constipation, 17 had HAEC, and 12 had both problems at different times. Twenty-seven patients were asymptomatic.
Of six patients with TZNR, one patient had constipation, one had HAEC, and two patients had both problems at different times. Two patients were asymptomatic. There were no statistically significant differences regarding the number of patients with HAEC, constipation, or asymptomatic presentation (p = 0.853, p = 0.960 and p = 0.360, respectively) between both groups. No patient required reoperation (Table 2).
Table 2 Distribution of 71 patients with Hirschsprung´s disease by type of neorectum and postoperative evolution
| Type of neorectum | ||||
|---|---|---|---|---|
| Normoganglionic (n = 65) | Transition zone (n = 6) | Number of patients | p-value | |
| Constipation | 9 (13%) | 1 (16.6%) | 10 | 0.960 |
| HAEC | 17 (26%) | 1 (16.6%) | 18 | 0.853 |
| HAEC and constipation | 12 (18%) | 2 (33.3%) | 14 | 0.818 |
| Asymptomatic | 27 (41%) | 2 (33.3%) | 29 | 0.304 |
*HAEC: Hirschsprung´s associated enterocolitis.
Discussion
HAEC and constipation remain two common postoperative problems in HD. In the present study, more than half of the patients had one or both, but we observed no difference in the frequency of these problems between patients with NNR or TZNR. These results suggest that HAEC and constipation cannot be attributed only to the TZ pull-through. However, it is undeniable that it is in the best interest of the patient to perform a complete resection of the aganglionic bowel and TZ bowel and perform a pull-through with the normoganglionic bowel.
Receiving an intraoperative result of the proximal margin as normoganglionic and, days later, receiving the final report as a TZ creates a dilemma. In this circumstance, the question is: Do we need to perform a reoperation to resect the bowel of the TZ? Based on the present study, the answer would be no. We suggest waiting and seeing the patient´s progress. If HAEC or constipation is present, medical treatment should be initiated in the same manner as patients with NNR who present with HAEC or postoperative constipation. Consistent with our findings and suggestions, Shankar et al.12 reported 10 patients (8.7% of a cohort of 114), and Ghosh et al.13 reported eight patients (16% of a cohort of 50) with TZ neorectum. Both authors reported successful outcomes with medical management, and no patients underwent reoperation. Furthermore, these authors suggested not to reoperate these patients due to the high incidence of fecal incontinence in reoperations.
It is recommended to resect an additional 5-10 cm from an intraoperatively reported full-thickness biopsy with ganglion cells without nerve trunk hypertrophy to avoid creating a neorectum in the TZ when performing a pull-through procedure in circumstances where the entire circumference of the proximal margin cannot be assessed with an intraoperative study14.
Another scenario is to evaluate a patient with HAEC or constipation after a pull-through who was operated on in another hospital, and the histopathology of the proximal margin is not known. In this situation, there is a possibility that the patient has an ANR. Ideally, the resected specimen should be requested to examine the proximal margin. If access to this tissue is unavailable, a full-thickness biopsy of the neorectum should be performed. This biopsy should be taken proximal to the anastomotic line, so its identification is essential (Fig. 5). A neorectal biopsy helps diagnose ANR by observing submucosal and myenteric plexuses without ganglion cells, truncal hypertrophy, and negative calretinin. It is very important to mention that if the biopsy is taken below the line of the anastomosis, that is, in the residual rectum, the report of "absence of ganglion cells" will be obtained. However, this result is a false positive.

Figure 5 Diagram of a normoganglionic neorectum showing the site where the neorectum biopsy should be taken above the anastomosis line.
According to the guidelines of the Hirschsprung Disease Interest Group, when a patient presents with HAEC or constipation after descent, it is necessary to rule out that the patient has ARN or TZNR. Consequently, these patients should undergo a biopsy of the neorectum. This group recommends a new pull-through if the neorectum biopsy shows a TZ because the specimen has ganglion cells, but there is hypertrophy of the nerve trunks. However, this recommendation was invalidated since 2016, when Kapur et al. demonstrated that submucosal nerve plexus hypertrophy in neorectum biopsies with ganglion cells should not be considered evidence for diagnosing TZNR15,16. Therefore, after the pull-through, biopsy of the neorectum is useful only to diagnose an aganglionic neorectum.
The goal of performing a new pull-through procedure justified by the diagnosis of TZNR should be to relieve HAEC, constipation, or both. If this decision is made, a critical step is to protect the entire anal canal to prevent fecal incontinence. Several authors have published their experience with reoperations in patients with TZNR diagnosed by neorectum biopsy. Unfortunately, most reports do not include postoperative functional outcomes. The few authors who have reported them mention improvement of HAEC or constipation, but the patients showed fecal incontinence17-29. Reoperation increases the likelihood of performing a low anastomosis, (i.e., an anastomosis across the anal canal). Damage to the anal canal is the main cause of fecal incontinence in Hirschsprung´s patients30-32. Furthermore, a redo pull-through does not guarantee the resolution of obstructive symptoms17-29. The evidence presented here suggests that medical management should be attempted in patients with HAEC or postoperative constipation before reoperation is proposed12,13.
The pull-through procedure requires an intraoperative histologic study to confirm that the descent is in a normoganglionic bowel. The pathologists experience is important to ensure that the intraoperative study is consistent with the final study. It has been reported that the concordance of the intraoperative report with the final report is 89%8,9; in this study, it was 91.5%. The literature reports an incidence of neorectum in the TZ of 18%18; in this study, it was 8.4%.
A limitation of our study is the difference in the number of patients in each group: there are more NNRs than TZNRs. This outcome is expected and even normal. Regardless of the sample size, there will always be more patients with NNR than TZNR in HD sigmoid rectum. Another limitation is that, despite the prospective or retrospective nature of this type of study, HD is a rare pathology with an incidence of 1:5,000 newborns14.
In order to create a homogeneous cohort, we only included patients with rectosigmoid HD, as this is by far the most common form that is primarily operated on and in which the proximal margin is examined intraoperatively. We excluded longer or total segments and syndromic HD, which have a higher incidence of HAEC compared to sigmoid rectus and non-syndromic HD33,34.
Finally, we conclude the following:
- HAEC and postoperative constipation occur in patients with rectosigmoid HD with normoganglionic neorectum and TZ neorectum
- Since there is no difference in the frequency of enterocolitis and constipation between patients with TZ pull-through and normoganglionic pull-through, these obstructive symptoms may have another cause
- Our results cannot be generalized to patients with long-segment HD, total colonic aganglionosis, and syndromic HD. Therefore, further studies should be performed to validate our findings.











 nova página do texto(beta)
nova página do texto(beta)