Introduction
Acute generalized exanthematous pustulosis (AGEP) is a severe cutaneous adverse reaction. AGEP is characterized by the sudden development of numerous millimetric, sterile, disseminated, non-follicular pustules on an erythematous-edematous base and is usually associated with fever and leukocytosis1. Drugs cause approximately 90% of cases; the remainder is caused by viral and bacterial infections and contact with mercury and spider bites2. Its worldwide incidence is 1 to 5 cases per million individuals per year. It is more frequent in adult women and rare in children2-4.
In 1968, Baker and Ryan published on five patients with no history of psoriasis who presented with pustular eruptions of sudden onset, rapid remission, and no recurrence. They named the pathology exanthematous pustular psoriasis, suspecting that drugs or infections were the triggers. In 1980, Beylot et al.5 introduced the term acute generalized exanthematous pustulosis. In 1991, Roujeau et al. initially described diagnostic criteria using the European Study of Severe Cutaneous Adverse Reactions (EuroSCAR)6:
Acute pustular rash
Fever > 38 oC
Neutrophilia with or without eosinophilia
Subcorneal or intraepidermal pustule on skin biopsy
Spontaneous resolution in less than 15 days3
In 2001, based on these criteria, Sidoroff et al. included a more detailed, rigorous, and specific scoring system, validated by the EuroSCAR study group5,6.
This study aims to encourage physicians, in general, and pediatricians and first contact physicians, in particular, to learn more about this rare disease in children. Although AGEP is a severe cutaneous adverse reaction, it is usually benign and uncomplicated. Therefore, a thorough understanding of this disease will prevent aggressive and inappropriate management.
Clinical case
We present the case of a 10-year-old female scholar with pruritic dermatosis associated with a fever of five days’ evolution. The patient reported that a week before the onset of the symptoms, she had had a transient, febrile upper respiratory tract infection resolved with symptomatic management. On examination, the patient was in good general condition, with a temperature of 39 ºC, 31.5 kg of weight, and 134.5 cm of height. The patient complained of pruritus and a burning sensation in the lesions distributed on the trunk, inguinal region, and proximal thighs (Figure 1A). The lesions consisted of numerous 1-3 mm small non-follicular pustules, some confluent, on an erythematous base (Figure 1B), with no involvement of the palms, soles, and mucous membranes. A skin biopsy was performed, and symptomatic management with oral hydroxyzine and an emollient cream was indicated. Two days later (at 7 days of evolution), pustules were no longer observed, but erythema and pruritus with residual desquamation persisted (Figure 1C). Laboratory studies reported the following results: culture for pustule bacteria, negative; immunoglobulin (Ig)G and IgM for herpes simplex type 1 and Tzanck’s test, negative; hemoglobin (Hb), 13.6 g/dL; hematocrit (HCT), 40.8%; platelets, 370,000/μL; leukocytes, 6,420/μL; neutrophils, 2,620/μL; lymphocytes, 2,630/μL; eosinophils, 460/μL; glucose, cholesterol, triglycerides, transaminases, uric acid, blood urea nitrogen (BUN), and creatinine levels were normal. Biopsy confirmed the diagnosis of AGEP (Figure 2). According to the EuroSCAR scale for AGEP, the following diagnostic scores are used for the diagnosis: 1-4, possible; 5-7, probable; 8-12, definitive. As the patient obtained a score of 11, the diagnosis of AGEP was definitive.
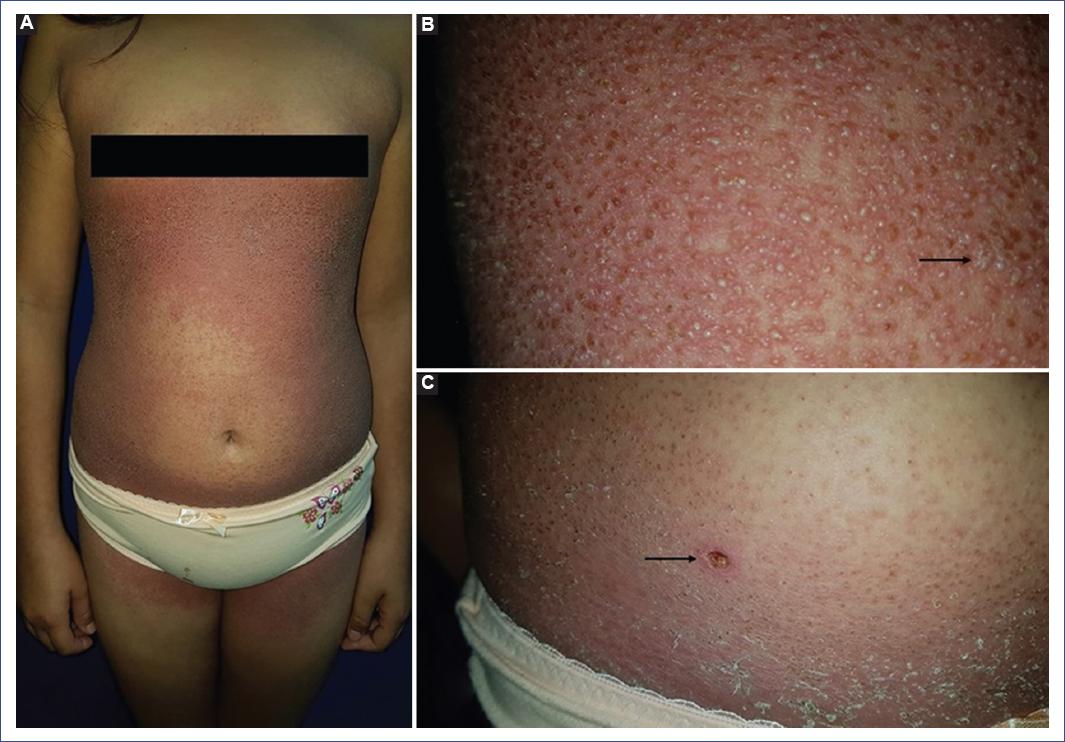
Figure 1 A: acute generalized exanthematous pustulosis. Dermatosis on trunk, inguinal region, and thighs. B: hundreds of non-follicular pustules on an erythematous base are observed in the lumbar region, some confluent (arrow). C: two days later, erythema persists, but pustules are no longer observed. Post-pustular desquamation is also observed. The arrow indicates the biopsy site.
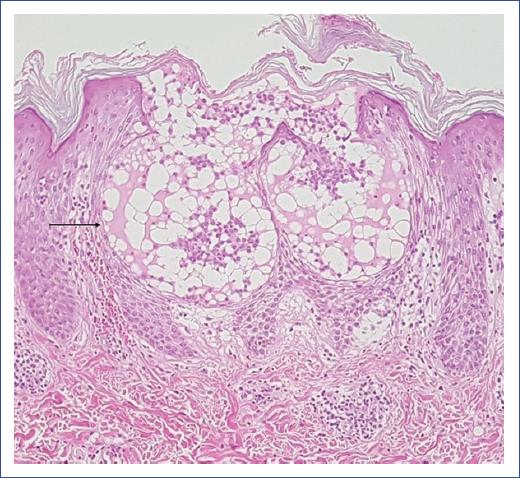
Figure 2 Acute generalized exanthematous pustulosis. Subcorneal blister in the stratum of Malpighi containing fibrin, few neutrophils, and eosinophils (arrow). Spongiosis and lymphocyte exocytosis are observed. Papillary and upper reticular dermis with mild lymphocytic inflammatory infiltrates and few eosinophils with perivascular distribution and intravascular neutrophils. Dilated capillaries with mild wall edema, some congestive with focal erythrocyte extravasation (hematoxylin-eosin stain, 10x).
Discussion
AGEP mainly affects adults and is infrequent in children. The estimated global incidence of this condition is 1 to 5 cases per million individuals per year. In contrast, it is approximately 1 case per million children per year7. Pediatric publications on AGEP are mainly case reports and a case series with few patients7-9. In a systematic review, RegiSCAR reported 49 pediatric cases up to 201410. AGEP is usually observed around the sixth decade of life, with a slight predominance in females. Its main risk factors are drug intake, the mean age of 50, and other comorbidities such as diabetes mellitus, psoriasis, and a history of drug hypersensitivity. Seventeen percent of individuals with AGEP have a previous history of psoriasis11,12.
Drug intake causes more than 90% of the reported cases of AGEP, most frequently antibiotics, mainly beta-lactams and macrolides3. Other drugs involved include aminoglycosides, sulfonamides, quinolones, antifungals, antimalarials, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), antihypertensives, calcium channel blockers, and antiepileptics11,13,14. The remaining 10% of the cases of AGEP have been associated with viral (enterovirus, adenovirus, parvovirus B19, cytomegalovirus, hepatitis B, Epstein Barr, among others), bacterial (Chlamydia pneumoniae, Escherichia coli, Mycoplasma pneumoniae, among others), and mycotic (pustulosis caused by fungi) infections, spider bites, mercury poisoning15,16, parasites, and food allergens, as well as radiotherapy, chemotherapy, and pregnancy11,13,14. It has been suggested that viral infections could be the most frequent trigger in the pediatric population2. The latency between drug exposure and the onset of AGEP varies from a few hours to 1 to 2 weeks, classically ranging from 48 to 72 hours; in the case of antimicrobials, it is usually less than 24 hours1,16.
Research studies on the pathophysiology have suggested that AGEP is a T-cell-mediated disease. Following drug exposure, specific CD4+ and CD8+ T cells are activated, proliferate, and migrate to the dermis and epidermis. CD8+ T cells induce apoptosis of keratinocytes within the epidermis by forming subcorneal and intraepidermal vesicles using perforin/B-granzyme and Fas ligand mechanisms. CD4+, CD8+ T cells, and natural killer (NK) cells have also been shown to express granulysin in different reactions to drugs or AGEP, suggesting that this substance may also play a role in pathogenesis. During the initial phase, vesicles formed from keratinocyte apoptosis contain mainly drug-specific CD4+ T cells and keratinocytes, which release large amounts of a potent neutrophil cytokine (CXCL8). CXCL8 transforms vesicles into sterile pustules by neutrophil chemotaxis1,17. Analysis of drug-specific CD4+ T cells from AGEP patients shows a predominant Th1 profile with increased production of interferon-gamma (IFN-g) and granulocyte/macrophage colony-stimulating factor (GM-SCF), which increases neutrophil survival by enhancing sterile pustule formation.
IFN-g and GM-SCF can induce the release of CXCL8 by keratinocytes, promoting neutrophil accumulation. Furthermore, CD4+ T cells of some AGEP patients have a Th2 profile with elevated production of IL-4 (interleukin 4) and IL-5 (a potent stimulator of eosinophil growth and proliferation), which explains the eosinophilia found in up to 30% of AGEP cases1. A higher level of IL-17 expression by neutrophils, mast cells, and macrophages and a lower level by T cells has been described in patients with AGEP, suggesting that innate cells may be involved in pathogenesis17. IL36RN gene mutations and increased CXCL8-mediated neutrophilic chemotaxis have been found in some patients with AGEP, suggesting their involvement in its pathogenesis7. The genetic predisposition for the development of AGEP is unknown; however, there might be a correlation between mutations of IL-36RN, which encodes the interleukin-36 receptor antagonist (IL-36Ra), and the development of generalized pustular eruptions after drug consumption. This suggests that patients with such a mutation are predisposed to develop AGEP 1,17. In addition, IL-36 Ra deficiency in some patients with AGEP appears to play a role in the increased expression of several proinflammatory cytokines and chemokines, such as IL-1, IL-6, IL-12, IL-23, IL-17, tumor necrosis factor-alpha (TNF-a) and CXCL8/IL-8, which enhances the recruitment and activation of neutrophils1.
In cases of AGEP of infectious etiology, there is no precise pathophysiological mechanism. However, it is hypothesized that infectious antigens could cross-react with drug antigens, act as haptens, or synergistically with some drugs, triggering the same immunologic reaction as in classical drug-induced AGEP16. Regarding the underlying pathogenesis, it has been proposed that drug-like infections lead to T-cell activation7.
AGEP manifests clinically as a cutaneous eruption of acute onset and is characterized by the appearance of numerous sterile, non-follicular pustules < 5 mm in diameter on an erythematous and edematous base. The dermatosis predominates on the trunk, upper extremities, and intertriginous regions, mainly the neck, axillae, and inguinal areas. However, it may be disseminated without affecting the face, palms, and soles, as in the present case. Occasionally, pustules may coalesce and lead to superficial collarette-shaped desquamation at the sites of the previous lesions18. The reaction is limited to less than 15 days after discontinuation of the causative agent or remission of the infection. It may be accompanied by fever, leukocytosis, neutrophilia, and sometimes eosinophilia, depending on the case. Systemic involvement with hepatic, renal, or pulmonary involvement has been described in 17% of patients17. Other symptoms include pruritus or burning sensation and mucosa involvement in up to 20% of cases, generally in severe forms and usually restricted to the oral mucosa or rarely to the conjunctivae1,16. Edema of the scrotum, hands, face, purpura, petechiae, vesicles, blisters and target lesions have been reported in 50% of patients11,17. In addition, severe atypical presentations of AGEP have occasionally been described. These extreme forms have been considered the overlap between AGEP and toxic epidermal necrolysis (TEN) or drug reaction with eosinophilia and systemic symptoms (DRESS). They may present with a clinical course similar to these conditions but with the histopathology of AGEP6,16,17.
The diagnosis of AGEP is based on clinical and histological criteria validated by the EuroSCAR study group3. A more precise evaluation based on morphology, course, and histopathological findings was proposed by Sidoroff et al. and validated by the EuroSCAR group. According to the score obtained, the case can be classified as possible (1 to 4 points), probable (5 to 7 points), or definitive (8 to 12 points)5,6,19. According to this scale, the reported case was diagnosed as definitive for AGEP, scoring 11 points.
Histopathology confirmed the clinical diagnosis. Usually, subcorneal or intraepidermal spongiform pustules, edema of the papillary dermis, perivascular infiltrates of neutrophils, and some eosinophils are observed (Figure 2). In addition, focal keratinocyte necrosis and leukocytoclastic vasculitis are occasionally detected17,20.
Patch tests are helpful when it is necessary to identify the drug involved4. A multicenter study showed that 58% of AGEP patients showed positive patch tests4,10; another study found positivity in 80%19. A negative test does not rule out the involvement of a particular drug. Dermoscopy with polarized light can be helpful: in the early stages, small, milky macules and papules, round globules, and sparsely scattered crusts on a pinkish/reddish background are observed21.
The differential diagnosis of AGEP should be made primarily with generalized pustular psoriasis of the von Zumbusch type. However, it is sometimes difficult to differentiate, as both may present with a similar clinical picture and unclear histopathology. In the latter, however, there is usually a previous history of psoriasis, fever, and a rash that persists longer, has a more generalized distribution with a tendency to recur, and can be fatal if not adequately treated11,17. The history, clinical picture, and histopathological findings can easily exclude other pustular eruptions of bacterial or fungal origin and neutrophilic dermatoses17. Differential diagnoses with drug-induced skin disorders such as DRESS syndrome, Stevens-Johnson syndrome, and NET should be considered in severe cases3. The most critical factor for differential diagnosis is the faster resolution time observed in AGEP17.
Although AGEP is benign and self-limited in most cases, it can present renal, hepatic, pulmonary, and bone marrow complications17. These complications are infrequent and occur mainly in the elderly, patients with comorbidities, or those with mucous membrane involvement1,11. Visceral involvement occurs in < 20% of cases, and amoxicillin is the most commonly implicated drug16. In these cases, it can cause death due to multiorgan dysfunction and disseminated intravascular coagulation, although mortality occurs in < 5% of cases1.
Cases have been reported of patients with AGEP presenting with systemic involvement: hepatomegaly, anemia, acute respiratory failure, hypotension, acute renal failure, cholestasis, and spinal cord involvement, as well as elevated liver function test values (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and gamma-glutamyl transferase), and increased urea and creatinine20. In addition, high absolute neutrophil count and C-reactive protein levels were associated with systemic organ involvement1.
There are no evidence-based treatment guidelines for the management of AGEP16. However, since its resolution is usually spontaneous, treatment is supportive, so topical corticosteroids, moisturizers, emollients, antipyretics, and oral antihistamines are frequently adequate15. The most critical measure is the suspension of the causative drug (or the remission of the infectious condition). With this, the cutaneous and even systemic involvement resolution occurs in < 15 days16, as in this case. In the pustular phase, moist dressings with antiseptic solutions and topical steroids can be used, while in the desquamative (post-pustular) phase, emollients could optimize the barrier function16,17. Antibiotics are counter-indicated unless there is superinfection of the lesions1,13. Medium and high potency topical corticosteroids are used for symptomatic relief of pruritus and inflammation22. Systemic corticosteroids are not usually necessary. Some authors consider them effective in cases of extensive cutaneous involvement (prednisone 0.5-1 mg/kg/day)3,13, although two cases of oral corticosteroid-induced AGEP have been reported17. The usefulness of cyclosporine and etanercept in patients resistant to corticosteroid treatment has also been described18. In cases of AGEP mimicking NET, intravenous immunoglobulin has been used16. Reintroducing the implicated drug should be avoided because of the risk of recurrence, usually with a more rapid onset13.
The prognosis of this disease is generally favorable, even if there is systemic involvement13. The condition resolves spontaneously approximately 10-15 days after discontinuation of the causative drug. Initially, pustules and fever disappear, followed by the collarette-shaped desquamation6. The same evolution is observed in cases of AGEP with infectious etiology, which is more frequent in children, as in the case described.
In conclusion, AGEP is a rare disease in pediatric patients. However, this condition should be considered a possible diagnosis in the presence of a sudden onset picture with numerous non-follicular pustules on an erythematous base and associated with fever. Pharmacological etiology is predominant, although AGEP is often associated with infections in children. The EuroSCAR scale facilitates the confirmation of the diagnosis. In most cases, treatment is supportive with an excellent prognosis. Knowledge of this pathology will improve the diagnostic and therapeutic approach to avoid aggressive and inappropriate management.











 nueva página del texto (beta)
nueva página del texto (beta)


