Introduction
Respiratory distress syndrome (RDS) continues to be a leading cause of neonatal mortality and morbidity in many countries worldwide1-4.
In the United States, mortality due to RDS decreased by 95% between 1970 and 2005. During this period, RDS dropped from being the first to the eighth leading cause of neonatal death5,6. Between 1970 and 1985, 75% of this decrease occurred when continuous positive airway pressure (CPAP) was initiated for neonates with RDS before surfactant therapy became available. Non-invasive use through a nasal cannula (nCPAP)7 began in 1974. Other improvements in care that contributed to reducing mortality were the development of ventilators designed for neonates (in the 90s), exogenous surfactants, and, more recently, the development of ventilators with gentler ventilation modes5.
In Mexico, mortality due to RDS decreased 46% from a rate of 2.6 to 1.4/1,000 live births between 1998 and 20178,9. In many hospital centers, RDS is no longer among the leading causes of death10,11. However, in the Instituto Mexicano del Seguro Social (IMSS), RSD is still the second leading cause of neonatal mortality nationwide12. Despite advances in its management, mortality due to RDS in Mexico is 13.5 times higher than in the United States (1.4/1,000 live births vs. 0.103/1,000 live births)13.
Furthermore, the decrease in RDS mortality is accompanied by an increase in bronchopulmonary dysplasia (BPD), a condition that is a frequent consequence in RDS survivors worldwide. Mechanical ventilation plays a predominant role in the generation of BPD14,15.
Early use of CPAP at birth reduces the need for mechanical ventilation and decreases the combined outcome of BPD or death16. According to some reports, the rate of BPD is two to three times higher than in developed countries17-19.
In Mexico, retinopathy of prematurity (ROP) is associated with mechanical ventilation, uncontrolled oxygen exposure, and prematurity. ROP is 2.4 times higher than in developed countries and is also the cause of infant blindness in 34% of those attending schools for the blind20,21.
Continuous flow devices can generate positive airway pressure: bubble CPAP, conventional ventilator-generated CPAP, and variable flow devices.
Bubble CPAP is the most efficient and inexpensive of these devices22. It consists of an air/oxygen mixer, a flowmeter, a servo-heated humidifier, an inspiratory circuit with a heating cable to reduce condensation, and an interface for its application in the neonate (nasal cannula or nasal mask) with its fixation system. It also has an expiratory circuit at its distal end connected to the pressure generator, which consists of a bottle with distilled water where the end of the expiratory course is submerged as many centimeters as centimeters of water pressure are desired to be generated. It is essential to have each one of its components for its correct operation (Figure 1).
Since the late 70s, bubble CPAP has been used in developing countries by adapting a nasal cannula and a circuit to a nebulizer.
Unfortunately, it is often misused: without the air/oxygen mixer because there is no mixer in each oxygen port, and there is only one mixer on mechanical ventilators. For approximately 15 years, bubble CPAP devices with all the required elements and safety measures have been marketed in Mexico, but their use is not widespread.
In the treatment guidelines for this disease, which have been developed in different countries, including Mexico, CPAP is the treatment of choice for stabilizing newborns. CPAP should be started early to reduce morbidity and mortality due to this disease and reduce the possibility of reintubation after extubation23-25. Since the equipment and supplies necessary for its use are suspected to be not adequately provided in the numerous neonatal care centers in the country, the Commission on CPAP and Best Practices for Ventilatory Support in Neonates of the National Federation of Neonatology of Mexico (Federación Nacional de Neonatología de México) decided to investigate the availability of CPAP equipment and supplies.
This study aimed to determine neonatal medical personnel’s perception of the availability of CPAP equipment and supplies in public and private secondary and tertiary level hospitals in Mexico.
Methods
To know the perception of the availability of equipment and supplies to provide nCPAP in Mexican hospitals with neonatal intensive care units (NICU), we developed a survey using Google forms. The link to answer the survey was sent to the presidents of the associations belonging to the Federación Nacional de Neonatología de México A.C. in each state of the country. They were asked to answer the questions related to the hospital(s) in which they worked and to send this link to their colleagues in public and private hospitals at the secondary and tertiary levels of care in the different health institutions in their city or state. One response per hospital was requested, and those who responded were followed up. Survey responses were received between June 16 and August 31, 2021.
The survey consisted of 27 questions. The demographic questions were about professional training (neonatologist, pediatrician, nurse, other), working hospital, level of care (public tertiary level, public secondary level, private tertiary level, private secondary level), affiliation institution (IMSS, Instituto de Salud para el Bienestar [INSABI], State or Municipal Secretariat, Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado [ISSSTE], PEMEX, SEDENA, SEMAR, or private), number of births/year, percentage of premature births, and state of the republic.
The dichotomous questions (with YES or NO answers) were the following:
Does the delivery room have CPAP?
Does the delivery room have an air/oxygen mixer?
Does the delivery room have a pulse oximeter?
Is there a CPAP for transfer?
Is the number of equipment to provide CPAP in the NICU sufficient for the demand for care?
The same questions were asked for the delivery room and intermediate care areas:
Do all CPAP devices have an air/oxygen mixer?
Is a complete tower to provide CPAP (CPAP equipment integrated into a rolling pedestal with all its components) available?
Regarding consumables, the questions were as follows:
1. Is the supply of nasal cannulae sufficient and timely?
The same question was asked for caps, circuits, CPAP generators, and surfactants.
Response options were based on an analog scale from 1 to 5 with extreme values of 1-never and 5-always. This variable was re-coded as a dichotomous variable, assigning the values 1 to 3 as NO and 4 to 5 as YES, to unify the presentation of the responses and facilitate analysis.
The nominal questions (with response options) were as follows:
-
What device do you primarily use to provide CPAP?
Bubble, ventilator-generated, variable flow, invasive/non-invasive ventilator.
-
What type of nasal cannula do you primarily use?
Hudson, Fisher & Paykel, Drager, or others.
-
What surfactant do you use?
Beractant or poractant.
Is CPAP equipment provided by purchase, commodate, or both?
Are consumables supplied by purchase, commodate, or both?
The usual surfactant application technique in your unit is INSURE, LISA, or conventional intubation-ventilation- extubation?
The percentage of healthcare staff (medical and nursing) formally trained in CPAP is less than 25%, 25-50%, 50-75%, or more than 75%?
One of the authors captured the responses and exported them to the SPSS statistical program (IBM Statistics SPSS 20) for descriptive and comparative analysis. The c2 test was performed to compare equipment and provision of CPAP supplies in public and private hospitals, by the level of care, and by the healthcare institution. A p-value < 0.05 was considered statistically significant. The percentage difference in responses between public and private hospitals was calculated. The 95% confidence intervals (95%CI) were calculated on the Statology.org website26.
The percentage of hospitals surveyed in relation to the total number of hospitals with public and private NICUs in Mexico was calculated according to data from the Dirección General de Información en Salud (DGIS) and the Instituto Nacional de Estadística y Geografía (INEGI), respectively27,28.
Results
A total of 241 surveys were received from 195 hospitals. Only one survey per hospital was included. We excluded 18 surveys sent more than once by the same respondent in the same hospital, 26 repeated surveys from the same hospital by different respondents, and two incomplete surveys (multiple or essential items unanswered). The exclusion of repeated surveys from the same hospital sent by different respondents was based on the following criteria: key or multiple unanswered items and inconsistencies with other responses from the same hospital. When more surveys from the same hospital remained after applying the above criteria, only one survey was randomly selected.
Surveys were received from 30 states in Mexico with a mean ± standard deviation (SD) of 6.5 ± 4.7 hospitals per state.
According to data from the DGIS, 368 public hospitals with NICUs are registered in Mexico23. A total of 195 hospitals were surveyed, representing 39% of those with NICUs.
From January 1, 2020, SSA hospitals and several hospitals of the State and Municipal Health Secretariats became part of INSABI, so they were grouped in this classification.
According to INEGI (health statistics in private facilities 2019), 47 private hospitals with NICUs are registered in Mexico24. Fifty private sector hospitals were included in the survey.
The distribution of the number and percentage of surveyed hospitals from different healthcare institutions and public and private hospitals according to the level of care is shown in Table 1.
Table 1 Surveyed hospitals classified by health institution and level of care
| Healthcare institution | n | % |
|---|---|---|
| State or Municipal Health Secretariat | 60 | 30.8 |
| Private | 50 | 25.6 |
| IMSS | 41 | 21.0 |
| ISSSTE | 19 | 9.7 |
| INSABI | 18 | 9.2 |
| PEMEX, SEDENA, SEMAR | 5 | 2.6 |
| IMSS Bienestar | 2 | 1.0 |
| Total | 195 | 100 |
| Level of care | n | % |
| Public | ||
| Tertiary level | 35 | 17.9 |
| Secondary level | 110 | 56.4 |
| Private | ||
| Tertiary level | 22 | 11.3 |
| Secondary level | 28 | 14.4 |
| Total | 195 | 100 |
IMSS: Instituto Mexicano del Seguro Social (Mexican Social Security Institute); INSABI: Instituto de Salud para el Bienestar (Health Wellness Institute); ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (Institute of Security and Social Services for State Workers); PEMEX: Petróleos Mexicanos (Mexican Petroleum); SEDENA: Secretaría de la Defensa Nacional (Secretary of National Defense); SEMAR: Secretaría de Marina (Secretary of the Navy).
The distribution according to the number of births/year was as follows: 78% of hospitals with < 5,000 births; 16% of hospitals with 5,000-10,000 births; 4% of hospitals with 10,000-15,000 births; and only 1% hospital with > 15,000 births.
The rate of prematurity was 8-10% in 38%; 10-12% in 29%; 12-14% in 16%; 15-16% in 5% and > 16% in 13% of the surveyed hospitals.
The professional training of the respondents was neonatologist in 90%, pediatrician in 8%, pediatric intensivist in 1%, nurse in 0.5%, and postgraduate (MSc) in 0.5%.
Regarding equipment in the delivery room, CPAP was found in 65%, pulse oximeter in 64%, CPAP with air/oxygen mixer in 36%, and CPAP for transport in 40%.
The CPAP device used in the delivery room was a CPAP bubble (60%), a T-piece resuscitator (26%), and a flow-inflated resuscitation bag with positive end-expiratory pressure (PEEP) control (13%).
Regarding which CPAP device was mainly used in all care areas, the most frequent was bubble CPAP (66%), followed by conventional ventilator applied (18%), invasive/non-invasive ventilator (7%), variable flow CPAP (6%), and spring-loaded valve device (3%).
On whether devices to provide bubble CPAP were complete, 37% reported that all CPAP had an air/oxygen mixer, and 47% reported that all CPAP had a complete bubble CPAP tower.
Concerning the sufficiency of equipment for the demand for care in the different neonatal care areas, 60% sufficiency was reported in the NICU, 62% in intermediate care, and 46% in the delivery room.
Supplies for CPAP devices were considered sufficient and timely in 43% to 51% of nasal cannulas, caps, circuits, and pressure generator bottles.
The most frequent method of acquiring equipment and supplies was purchase (74% and 77%, respectively), followed by commodate (5% and 4%, respectively), and both in approximately 20%.
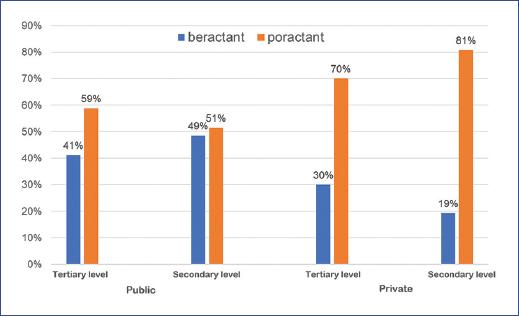
Surfactant supply was rated as sufficient and timely in 71% of hospitals. The surfactant types supplied were poractant (59%) and beractant (41%).
Regarding the usual surfactant delivery technique, the following was observed: intubation, surfactant, extubation (INSURE) in 55%; intubation, invasive ventilation, surfactant, conventional extubation in 31%; less invasive surfactant administration (LISA) in 12%.
The preferred interface was Hudson tips (58%); F&P, Dräger, and other tips (22%); Infant Flow (8%); F&P nasal mask (6%); and others (5%).
Regarding formal CPAP training, 5% of the hospitals had trained > 75% of the nursing staff, and 11% of the units had trained at least 75% of the physicians.
When comparing public and private hospitals regarding the availability of CPAP equipment and consumables, a statistically significant difference was observed: private hospitals showed greater availability of equipment and consumables in the delivery rooms and NICUs (Table 2).
Table 2 Continuous positive airway pressure (CPAP) equipment and supplies availability in public and private hospitals
| Availability of equipment and consumables | Hospitals | Difference (%) | 95% CI* | p-value | |
|---|---|---|---|---|---|
| Private (n = 50) | Public (n = 145) | ||||
| CPAP in the delivery room | 79.6% | 60.2% | 19.4% | [0.0568, 0.3312] | 0.014 |
| Mixer in CPAP devices | 51.2% | 30.6% | 20.6% | [0.0484, 0.3636] | 0.017 |
| Pulse oximeter in the delivery room | 81.6% | 57.9% | 23.7% | [0.1029, 0.3711] | 0.003 |
| Complete CPAP bubble tower | 77.6% | 36.3% | 41.3% | [0.2734, 0.5526] | <0.001 |
| All CPAP have a mixer | 51.0% | 32.9% | 18.1% | [0.0227, 0.3393] | 0.023 |
| Sufficient CPAP devices in NICU | 83.7% | 52.1% | 31.6% | [0.1853, 0.4467] | <0.001 |
| Adequate and timely nasal cannula supply | 75.5% | 36.3% | 39.2% | [0.2494, 0.5346] | <0.001 |
95%CI, confidence interval for differences; CPAP: continuous positive airway pressure; NICU: Neonatal Intensive Care Unit.
The primary device to provide CPAP at all levels was bubble CPAP, followed by conventional ventilator, invasive/non-invasive ventilator, variable flow, and spring-loaded valve (Figure 2).
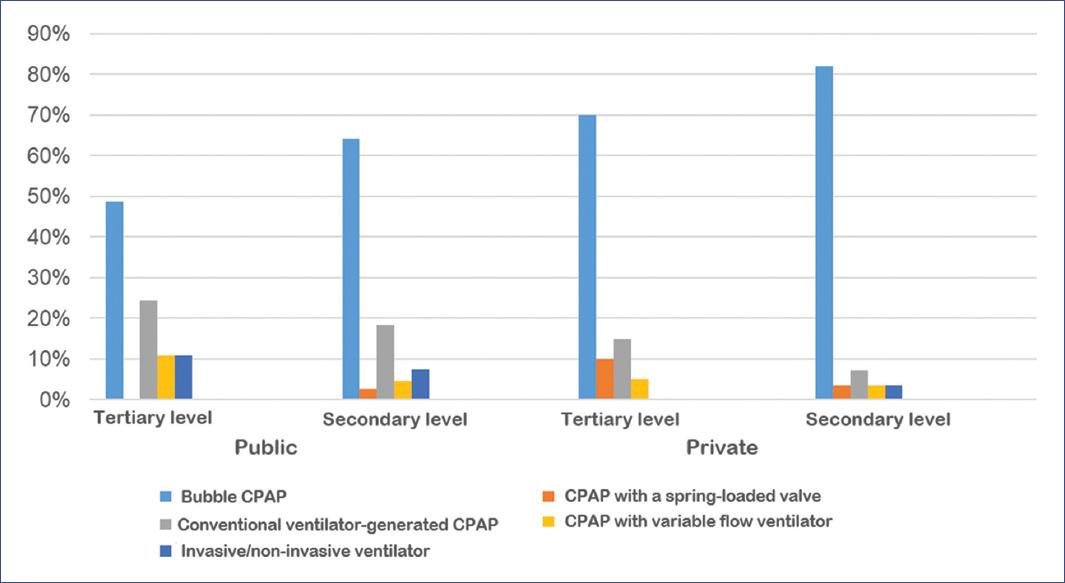
Figure 2 CPAP (continuous positive airway pressure) device mainly used according to the level of care.
The most frequently available surfactant in public and private secondary and tertiary level hospitals was poractant compared to beractant (p = 0.037) (Figure 3).
The most frequent surfactant application method was INSURE in public and private secondary and tertiary level hospitals, followed by invasive ventilation-intubation-conventional extubation and, less frequently, LISA (Figure 4).
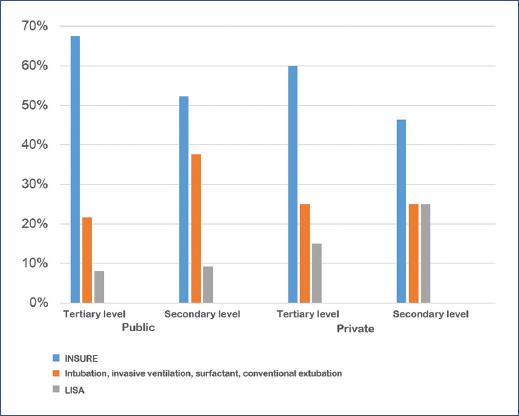
Figure 4 Method of surfactant application by the level of care. INSURE: intubation surfactant extubation; LISA: less invasive surfactant administration.
The availability of equipment and supplies by the level of care in public and private hospitals are shown in Table 3. The results of responses by the level of care in public and private hospitals are shown in Table 3.
Table 3 Availability of equipment and supplies by the level of care in public and private hospitals
| Level of care | Private | Public | p-value | ||
|---|---|---|---|---|---|
| Tertiary (n = 22) | Secondary (n = 28) | Tertiary (n = 35) | Secondary (n = 110) | ||
| Availability of equipment in the delivery room | |||||
| CPAP available in the delivery room | 86.4% | 78.6% | 66.7% | 57.7% | 0.022 |
| Air/oxygen mixer in the delivery room | 81.0% | 30.9% | 43.5% | 25.3% | < 0.001 |
| Oximeter available | 95.5% | 71.4% | 66.7% | 55.2% | 0.003 |
| CPAP available for transfer | 81.8% | 46.4% | 43.8% | 43.1% | 0.010 |
| Availability of CPAP in the neonatology wards | |||||
| Bubble CPAP with all its components | 95.6% | 67.9% | 37.1% | 34.5% | < 0.001 |
| All CPAP has an air/oxygen mixer | 77.3% | 35.7% | 48.6% | 26.4% | < 0.001 |
| Sufficiency of equipment by care area | |||||
| In the NICU | 95.5% | 75.0% | 68.6% | 46.4% | < 0.001 |
| In the intermediate care unit | 95.5% | 78.6% | 71.4% | 47.3% | < 0.001 |
| In the delivery room | 77.3% | 60.7% | 55.6% | 32.7% | < 0.001 |
| Adequate and timely supplies | |||||
| Nasal cannulas | 95.5% | 64.3% | 54.3% | 29.1% | < 0.001 |
| Caps | 95.5% | 67.9% | 51.4% | 23.9% | < 0.001 |
| Circuits | 100% | 64.3% | 62.9% | 33.6% | < 0.001 |
| CPAP bottle | 100% | 66.9% | 61.3% | 35.8% | < 0.001 |
| Surfactant | 100% | 67.9% | 82.9% | 62.7% | 0.002 |
CPAP: continuous positive airway pressure; NICU: Neonatal Intensive Care Unit.
There was a significant difference in the availability of equipment and consumables among healthcare institutions. Private hospitals had higher availability of equipment and consumables. Among public sector hospitals, State or Municipal Secretariats, INSABI, Pemex, and ISSSTE had better availability of equipment and consumables than IMSS. Because IMSS Bienestar received responses only from two hospitals, several answers appear as 0 (Table 4).
Table 4 Availability of equipment and consumables in each healthcare institution
| Health institution | Private (n = 50) | St-Mun (n = 60) | INSABI (n = 18) | ISSSTE (n = 19) | P-S-S (n = 5) | IMSS (n = 41) | IMSS-B (n = 2) | p-value |
|---|---|---|---|---|---|---|---|---|
| Availability of equipment in the delivery room | ||||||||
| CPAP | 82.0% | 73.6% | 64.3% | 42.1% | 20.0% | 53.8% | 0.0% | 0.001 |
| Devices have an air/oxygen mixer | 54.5% | 39.1% | 50.0% | 30.8% | 25.0% | 9.1% | 0.0% | 0.003 |
| Oximeter | 82.0% | 69.8% | 57.1% | 52.6% | 80.0% | 43.6% | 0.0% | 0.005 |
| CPAP for transfer | 62.0% | 59.3% | 52.9% | 10.5% | 20% | 35.9% | 0.0% | < 0.001 |
| Availability of CPAP in the neonatology wards | ||||||||
| Bubble CPAP with all its components | 80.0% | 39.0% | 55.6% | 42.1% | 40.0% | 14.6% | 50.0% | <0.001 |
| All CPAP have an air/oxygen mixer | 54.0% | 35.0% | 38.9% | 36.8% | 0.0% | 26.8% | 0.0% | 0.058 |
| Sufficiency of CPAP equipment | ||||||||
| In the NICU | 84.0% | 61.7% | 38.9% | 57.9% | 60.0% | 38.9% | 50.0% | 0.012 |
| In the intermediate care unit | 86.0% | 61.7% | 44.4% | 63.2% | 100% | 34.1% | 50.0% | < 0.001 |
| In the delivery room | 68.0% | 49.1% | 35.7% | 44.4% | 20.0% | 23.1% | 0.0% | 0.001 |
| Adequate and timely supplies | ||||||||
| Nasal cannulas | 78.0% | 45.0% | 44.4% | 26.3% | 60.0% | 19.5% | 0.0% | < 0.001 |
| Caps | 80.0% | 36.7% | 50.0% | 27.8% | 20.0% | 17.1% | 0.0% | < 0.001 |
| Circuits | 80.0% | 46.7% | 50.0% | 36.8% | 60.0% | 29.3% | 0.0% | < 0.001 |
| CPAP bottle | 82.0% | 49.2% | 56.3% | 31.6% | 80.0% | 25.6% | 0.0% | < 0.001 |
| Surfactant | 82.0% | 73.3% | 50.0% | 68.4% | 100% | 63.4% | 50.0% | 0.099 |
CPAP: continuous positive airway pressure; IMSS: Instituto Mexicano del Seguro Social; IMSS-B: IMSS Bienestar; INSABI: Instituto de Salud para el Bienestar; ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado; NICU: Neonatal Intensive Care Unit; P-S-S, PEMEX (Petróleos Mexicanos)-Secretaría de la Defensa Nacional-Secretaría de Marina; St-Mun: State or Municipal Health Secretariat.
The distribution of the devices mainly used to provide CPAP by healthcare institutions is shown in Figure 5. In all institutions, the primary device used was bubble CPAP, followed by CPAP supplied by the conventional ventilator, invasive/non-invasive ventilator, variable flow CPAP, and spring-loaded valve. The proportion varied from one institution to another.
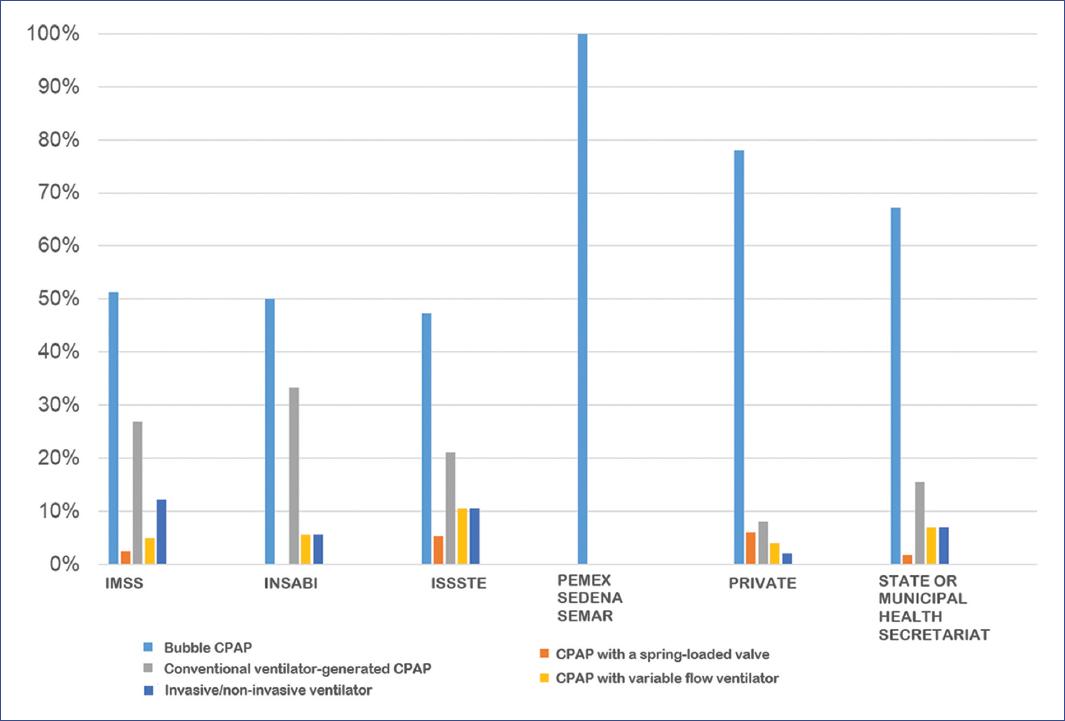
Figure 5 CPAP (continuous positive airway pressure) device distribution by healthcare institutions.IMSS: Instituto Mexicano del Seguro Social; INSABI: Instituto de Salud para el Bienestar; ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado; PEMEX: Petróleos Mexicanos; SEDENA: Secretaría de la Defensa Nacional; SEMAR: Secretaría de Marina.
The type of surfactant available, its distribution (Figure 6), and its method of administration (Figure 7) are shown for each healthcare institution.

Figure 6 Type of surfactant available and its distribution by healthcare institutions.IMSS: Instituto Mexicano del Seguro Social; INSABI: Instituto de Salud para el Bienestar; ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado; PEMEX: Petróleos Mexicanos; SEDENA: Secretaría de la Defensa Nacional; SEMAR: Secretaría de Marina.

Figure 7 Method of surfactant administration by healthcare institutions.IMSS: Instituto Mexicano del Seguro Social; INSABI: Instituto de Salud para el Bienestar; INSURE: intubation surfactant extubation; intub + surf + MV: intubation-surfactant-mechanical ventilation; ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado; LISA: less invasive surfactant administration; PEMEX: Petróleos Mexicanos; SEDENA: Secretaría de Defensa Nacional; SEMAR: Secretaría de Marina.
Discussion
Some limitations of the present study are that only one response per hospital was requested, which limits the representativeness of the perception of the availability of equipment and supplies; however, responses were reasonably consistent among health institutions and levels of care. Fifty private hospitals with NICUs were reported, while INEGI reports only 47; this means that some neonatology services providing intensive care to neonates with no official NICU registry were cataloged as having one. In addition, the survey did not define the criteria for assigning whether the hospital was a secondary or tertiary care setting, leaving it to the informant’s judgment. Furthermore, the results from IMSS Bienestar (two) and PEMEX-SEDENA-SEMAR (five) hospitals surveyed should be interpreted with caution due to the small sample size.
Improvements in the quality of newborn care, including nCPAP training, bubble CPAP equipment, and adoption of best care practices (prenatal steroids, CPAP from birth, early surfactant, avoidance of unnecessary intubation, early extubation) have an impact on reducing bronchopulmonary dysplasia, sepsis, neonatal ventilation, reduced surfactant use, and reduced ROP29-31. These interventions have been evaluated as cost-effective in developed and developing countries32-38.
In Mexico, there is a significant disparity in the availability of CPAP equipment and consumables between public and private sector hospitals and the different institutions surveyed, leading to the assumption that availability depends on economic and administrative factors.
The equipment to provide bubble nCPAP, the most commonly used device for this purpose, is not included as complete equipment in the basic list of medical instruments and equipment in the health sector39,40. The lack of an adequate mixer and humidifier exposes the neonate to the risk of short-and long-term morbidity and mortality, and the physician and the healthcare institution to malpractice, as it contravenes patient care and safety standards. Only the nasal cannula and circuit are included in the basic table of consumables41.
The increased availability of equipment and consumables in private hospitals could be related to the increase, in recent years, in the use of major medical health insurance among the middle and upper-class population, which has allowed private hospitals to have resources for equipment and consumables for their NICUs.
The purchasing and resource prioritization schemes could also explain the difference between IMSS and other public hospitals.
The catastrophic expense insurance granted resources to public hospitals accredited by the SSA and the State or Municipal Health Secretariats to care for newborns with respiratory failure and prematurity. To be accredited, the hospital had to meet quality standards regarding facilities, personnel, equipment, and organization to care for neonates with this pathology42.
In IMSS, equipment acquisition is programmed annually and adjusted according to the availability of resources and priorities. The disadvantage of this scheme is that the needs of a critical and priority health area compete with the multiple needs of the entire hospital, so the limited resources often do not reach where they are most needed.
Mortality and morbidity associated with RDS are still relevant problems in Mexico. Consequently, it would be advisable for public health institutions to improve their mechanisms for acquiring equipment and supplies to address priority areas with a high impact on morbidity and mortality.
Public health sector hospitals and institutions have an excellent opportunity to optimize resources by acquiring equipment for non-invasive ventilatory support of the neonate, prioritizing CPAP bubble complete equipment. In parallel, improvements in neonatal care are required with the training of multidisciplinary perinatal care personnel and the adoption of better care practices.
The initial cost of investing in this nCPAP equipment and supplies can be considered a significant saving due to a future decrease in morbidity and time of care in these infants, as well as a significant decrease in mortality associated with this condition—which should not be underestimated.
On this basis, there is an excellent opportunity for public and private hospitals to improve the availability of complete bubble CPAP devices for neonatal non-invasive ventilatory support and training of healthcare personnel in the adoption of best practices for non-invasive ventilatory support and less invasive surfactant application to have an impact on RDS mortality and associated morbidities, such as BPD and ROP.











 nueva página del texto (beta)
nueva página del texto (beta)