Introduction
The administration of intravenous fluids is a common practice in intensive care. In critically ill patients, adequate volume administration can improve systemic perfusion in different organs and tissues, thus preventing multiorgan dysfunction and death. However, this practice is often prolonged for extended periods without apparent benefit to the patient1. The cause of fluid overload (FO) in critically ill patients is often multifactorial. Sepsis, postoperative states, burns, or ischemia-reperfusion injury increase vascular permeability, capillary leakage, and interstitial edema.
Patients undergoing cardiac surgery with extracorporeal circulation frequently present hemodynamic instability, requiring acute administration of intravenous fluids to prevent and correct arterial hypotension. Low cardiac output syndrome and capillary leak syndromes produce interstitial edema and relative hypovolemia. Furthermore, hypovolemia may result from excessive ultrafiltration during extracorporeal circulation or postoperative bleeding. On many occasions, the administration of solutions and blood products in large quantities is required2.
Moreover, cardiac surgery patients also present increased vascular permeability secondary to the presence of endothelial inflammation. This inflammation is due to the release of cytokines and interleukins produced by the passage of blood through a circuit without endothelium during extracorporeal circulation, predisposing the patient to develop FO easily3.
Acute kidney injury occurs in 30% of patients and increases to 50% in neonates. This complication is mainly due to acute tubular injury and activation of the renin-angiotensin-aldosterone axis. Activation of this axis increases the release of antidiuretic hormone, producing sodium and water retention in the tissues with secondary hypervolemia. Angiotensin produces renal vasoconstriction, compromising renal perfusion and significantly decreasing glomerular filtration. All these factors predispose to the development of FO, impairing patient prognosis4,5. Some studies associate FO (> 7% in the first 48 hours) with increased intensive care unit stay6,7.
Similarly, excess fluids negatively affect patient oxygenation, increasing the oxygenation index (OI), a marker of restrictive pulmonary physiology. An elevated OI influences the duration of mechanical ventilation8,9.
The main objective of this study was to identify the frequency and degree of FO in patients undergoing cardiovascular surgery with extracorporeal circulation, as well as the association between the degree of FO and OI, days of mechanical ventilation, hospital stay, and mortality.
Methods
Study design
We conducted a longitudinal study of pediatric patients undergoing heart surgery with extracorporeal circulation. The study was conducted in the Pediatric Cardiac Intensive Care Unit (PCICU), Instituto Nacional de Pediatría, from July 2018 to December 2019. Before surgery, patients with the following conditions were excluded: clinical data of FO (manifested by soft-tissue edema, pleural effusion, or ascites), assisted mechanical ventilation, and presence of acute kidney injury (AKI).
Surgery was performed by median sternotomy and extracorporeal circulation pump in all cases. A Tenckhoff catheter was placed for fluid management at the end of surgery. All patients received the same fluid management protocol, consisting of 30% of Holliday Segar requirements in the first 48 to 72 hours10, followed by total parenteral nutrition of 40-80 ml/kg. Peritoneal dialysis at 1.5% was started at 10 ml/kg with inflow-by-outflow baths from admission.
Data collection
Data were collected in Microsoft Office 2016 Excel format. Demographic data collected were age, sex, procedure, and surgical complexity (RACHS classification)11. Intraoperative data collected were extracorporeal circulation time, aortic clamp time, circulatory arrest time, and temperature (°C). Postoperatively, data collected were the weight on admission, daily weight over the following 72 hours, and the percentage of FO accumulated every 24 hours during the 72 hours after surgery.
The percentage of FO was calculated with the following formula12:
Other variables collected every 24 hours were OI (mean airway pressure x inspiratory oxygen fraction x 100 divided by arterial oxygen pressure), inotropic score13, and AKI (AKI classification)14. In addition, the following outcome variables were collected: days of PCICU stay, days of mechanical ventilation, and mortality, both within the unit and 30 days postoperatively.
Statistical analysis
Analyses were performed with the statistical package STATA version 17.0 (StataCorp). Qualitative variables were presented as frequencies and proportions, while quantitative variables were presented as median and interquartile ranges. For the analysis, the population was divided into two groups according to the FO level: patients who presented FO ≥ 5% in any of the three measurements during the first 72 postoperative hours and patients with no FO or FO ≤ 4%.
The χ2 and Mantel-Henzell tests were used to identify associations between qualitative variables. The Kruskal-Wallis nonparametric test was used to identify differences between medians. Spearman's test was used in the case of correlations between quantitative variables. In all cases, a p-value < 0.05 was considered statistically significant. Outcome variables were dichotomized to identify confounding factors by multivariate analysis with logistic regression, for which odds ratio (OR) and 95% confidence intervals (CI) were presented.
Results
We included 130 patients who underwent heart surgery with extracorporeal circulation from July 2018 to December 2019. Sixty-one patients (47%) were female, and 69 (53%) were male. Twenty-one patients (16%) had FO ≥ 5% during the study period. Patients with FO ≥ 5% were younger and weighed less than those with FO < 5%. More than two-thirds of patients with FO ≥ 5% had high surgical complexity (RACHS ≥ 3). Also, extracorporeal circulation time was longer in the group with FO ≥ 5% (159 min vs. 109 min, p = 0.003) (Table 1).
Table 1 Sociodemographic and surgical characteristics
| Total (n = 130) | FO ≥ 5%* (n = 21) | FO < 5% (n = 109) | p-value | |
|---|---|---|---|---|
| Male | 69 (53%) | 12 (57%) | 57 (52%) | 0.683 |
| Female | 61 (47%) | 9 (43%) | 52 (48%) | |
| Age in months, median (IQR) | 16 (6-55) | 4 (1-11) | 22 (8-59) | < 0.001 |
| Weight in kg, median (IQR) | 8 (4.4-14) | 3.4 (3-4.8) | 9.5 (5.4-15) | < 0.001 |
| Age group | ||||
| < 30 days | 3 (2.3%) | 2 (67%) | 1 (33%) | 0.035 |
| 1-11 months | 54 (42%) | 15 (28%) | 39 (72%) | 0.026 |
| 1-4 years | 44 (34%) | 2 (5%) | 42 (95%) | 0.666 |
| ≥ 5 years | 29 (22%) | 2 (7%) | 27 (93%) | ___** |
| RACHS ≥ 3 | 51 (39%) | 16 (76%) | 35 (32%) | < 0.001 |
| RACHS | ||||
| 1 | 21 (16%) | 0 (0%) | 21 (100%) | 0.317 |
| 2 | 58 (44%) | 5 (9%) | 53 (91%) | ___** |
| 3 | 19 (15%) | 4 (21%) | 15 (79%) | 0.212 |
| 4 | 28 (22%) | 10 (36%) | 18 (64%) | 0.005 |
| 5-6 | 4 (3%) | 2 (50%) | 2 (50%) | 0.059 |
| Extracorporeal circulation time (min) | 119 (77-171) | 159 (122-213) | 109 (72-164) | 0.003 |
FO, fluid overload; IQR, interquartile range; RACHS, risk adjustment for congenital heart surgery.
*Includes patients with FO ≥ 5% in any of the three measurements taken during the first 72 hours postoperatively.
**Reference group.
Patients with FO ≥ 5% were 11.6 times more likely to require a high inotropic score (≥ 10) during the study period, regardless of the RACHS-1 surgical risk scale. Concerning respiratory variables such as OI, patients with FO ≥ 5% showed 3.5 times higher odds of an OI ≥ 8. Consequently, their stay in the PCICU was longer, and they required more mechanical ventilation time. Mortality in the study population was 5%, representing seven patients, six of whom had an overload > 5%. Patients with significant FO were 89 times more likely to die than patients with no or < 4% overload (Table 2).
Table 2 Postoperative evolution and FO
| Total (n = 130) | FO ≥ 5% (n = 21) | FP < 5% (n = 109) | p-value | Raw OR (95% CI) | Adjusted OR* (95% CI) | |
|---|---|---|---|---|---|---|
| PCICU stay (days) median (IQR) | 7 (4-13) | 23 (13-31) | 6 (4-9) | < 0.001 | — | — |
| PCICU stay (≥ 7 days) | — | 20 (95%) | 49 (45%) | < 0.001 | 24.5 (3-189) | 16.6 (2-135) |
| VMS time (days) median (IQR) | 6 (2-10) | 15 (9-20) | 4 (1-7) | < 0.001 | — | — |
| VMS time (≥ 7 days) (n = 78) | — | 17 (85%) | 16 (28%) | < 0.001 | 14.8 (3.8-57) | 11.5 (2.7-48) |
| Inotropic score median (IQR) | 8 (4-20) | 28 (14-56) | 6.5 (3-14) | < 0.001 | — | — |
| Inotropic score (≥ 10, n = 105) | — | 19 (91%) | 32 (38%) | < 0.001 | 15.4 (3.4-70) | 11.6 (2.3-58) |
| Oxygenation index median (IQR) | 6.5 (4.5-8) | 7 (6-13) | 5 (4-8) | < 0.001 | — | — |
| Oxygenation index (≥ 8, n = 72) | — | 65% (13) | 27% (14) | 0.006 | 5 (1.6-15) | 3.5 (1.07-11.7) |
| Mortality | 7 (5%) | 6 (29%) | 1 (0.9%) | < 0.001 | 43 (4.8-384) | 89 (4.3-1813) |
CI, confidence interval; FO, fluid overload; IQR, interquartile range; OR, odds ratio; PCICU, Pediatric Cardiac Intensive Care Unit; VMS, ventilatory mechanical support.
*Adjusted for age in months, sex, and RACHS (risk adjustment for congenital heart surgery).
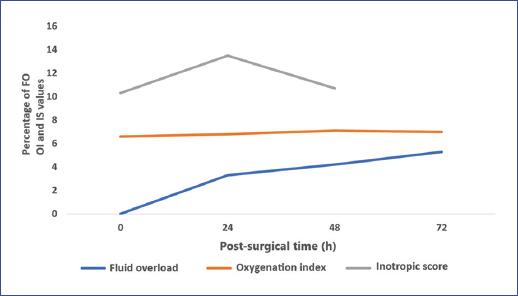
A positive correlation was found between inotropic score, OI, and maximum peak FO in the first 72 hours. The top peak FO during the study was 5 ± 4.3%, lower in the first 24 hours (3.3 ± 2.1%) and higher at 72 hours (5.3 ± 4.8%) (Table 3). Similarly, an increase in the inotropic score at 24 hours was observed parallel to the rise in FO (Figure 1).
Table 3 Correlation of fluid overload with oxygenation index and inotropic score.
| Fluid overload | Peak overload (mean ± SD) | Oxygenation index | Inotropic score | ||
|---|---|---|---|---|---|
| Spearman's coefficient | p-value | Spearman's coefficient | p-value | ||
| General | 5 ± 4.3% | 0.45 | 0.001 | 0.63 | < 0.001 |
| 24 h | 3.3 ± 2.1% | 0.11 | 0.473 | 0.59 | < 0.001 |
| 48 h | 4.2 ± 3.4% | 0.35 | 0.056 | 0.53 | 0.001 |
| 72 h | 5.3 ± 4.8% | 0.51 | 0.004 | — | — |
FO, fluid overload; SD, standard deviation.
Discussion
FO in critically ill patients remains a concern for critical care physicians due to its association with a less favorable outcome. Excessive fluid intake and AKI, frequently present in critically ill patients, lead to excessive fluid accumulation in the tissues, increasing the risk of FO.
Our study found an incidence of FO of some level in 44% and FO ≥ 5% in 16%, consistent with other authors such as Seguin et al.6, who found FO in 33.6%.
In the present study, the mean maximum peak of FO was 5% in the first 72 hours postoperatively. Seguin et al. reported a mean maximum peak of 7.4 ± 11.2% during the first 24 hours6, whereas we found 3.3 ± 2.1%. Furthermore, the mean FO at 72 hours was 5.3 ± 4.7%, lower than that described by Valentine et al.15 (8.5 ± 10.5%) or by Sinitsky et al.9 (7.2%) at 48 hours. In another study, Hassinger et al. found that the FO was 5% in the first 24 hours16. These differences can be explained by the use of early dialysis in the first 24 hours of the postoperative period as part of the PCICU fluid management protocol, especially in patients with complex heart disease.
Here, we also observed that age < 10 months and weight < 5 kg were factors associated with FO, as well as high surgical complexity (RACHS-1 ≥ 3). Sinitsky et al. also detected age as a risk factor and the severity scale using PIM2 (pediatric mortality index)9. This relationship between age, surgical complexity, and FO can be explained because the more complex the heart disease, the earlier the surgery. Hence, patients usually have greater hemodynamic instability and a higher risk of bleeding, requiring the administration of intravenous fluids to restore systemic perfusion. Other studies suggest the presence of cyanogenic heart disease (p = 0.03) and the administration of excess fluids in the first 6 hours (p = 0.0001) as risk factors6.
Pumping time was also longer in patients with FO ≥ 5%, with a median of 159 min (122-213), similar to those published by Seguin et al. of 138.9 ± 62.1 min (median of 134 min)6. These findings support the fact that the longer the extracorporeal circulation time, the greater the release of inflammatory mediators that produce increased vascular permeability and, consequently, the greater the need for intravenous fluid administration.
The inotropic score was also higher (> 10) in patients with FO ≥ 5%, similar to those reported in the meta-analysis conducted by Bellos et al. in 3111 patients17. In this meta-analysis, patients with cumulative FO rapidly developed cardiac dysfunction with a higher need for inotropic support (r = 0.7, p < 0.01)18. Volume overload negatively affects cardiac function in a heart stunned by using an extracorporeal circulation pump, requiring higher doses of inotropic drugs to preserve cardiac output.
Regarding respiratory variables, we found a significant correlation between OI and the percentage of FO ≥ 5% (p = 0.001) and between the maximum cumulative percentage of FO (coefficient = 0.45, p = 0.001), similar to that reported by Sinitsky et al. at 48 hours (coefficient = 0.318, p < 0.001)9.
In our population, patients who spent more time on mechanical ventilation had a higher percentage of cumulative FO (p = 0.001), a result similar to that reported by Bellos et al.17, showing a direct relationship between the overload index and days on mechanical ventilation (coefficient = 0.53, p = 0.001). Sampaio et al. also reported a direct relationship (coefficient = 0.54, 95%CI 0.38-0.66, p = 0.001) between peak FO and days of mechanical ventilation and soft tissue edema or pleural effusions on chest X-rays (p = 0.03)18.
Intensive Care Unit stay was longer in patients with FO ≥ 5% (p < 0.001), while Arikan et al. reported that FO > 15% was a risk factor for more extended ICU stay (p = 0.008)8. In the multicenter study by Bellos et al., a significant correlation was reported between ICU stay (χ2 = 63.69, p = 0.0001) and hospital stay (χ2 = 18.84, p = 0.0001).17
Several studies in critically ill pediatric patients have found FO to be an independent risk factor for mortality. For example, Goldstein et al. studied 116 patients with multiorgan dysfunction secondary to sepsis or cardiogenic shock. These authors found a higher survival (p < 0.03) in patients with a lower %FO (14.2 ± 15.9) than those with a higher %FO (25.4 ± 32.9)19.
In neonatal cardiac surgery patients, FO ≥ 16% on the third postoperative day was an independent risk factor for an undesirable outcome, such as cardiac arrest (p = 0.0001), increased hospital stay (p = 0.04), and the need for surgical re-exploration (p = 0.0001)20.
In our study, seven patients died, representing 5% of the total; six belonged to the FO ≥ 5% group (p < 0.0001). Sutherland et al. and Hayes et al. showed a direct relationship between FO and mortality21,22. The former found that for each 1% increase in FO, mortality increased by 3% (OR 1.03, 95%CI 1.01-1.05). When patients showed %FO > 20, the OR for mortality was 8.5 (95%CI 2.8-25.7).21
Some strategies to reduce systemic inflammation after extracorporeal circulation are steroids and ultrafiltration. Both aim to reduce interleukins and cytokines that act as inflammatory mediators and are responsible for the increased vascular permeability that results in significant interstitial edema. Other strategies used in the ICU, such as water restriction of up to 25% of maintenance fluids in the first 24 hours and diuretics and kidney replacement therapies, such as early peritoneal dialysis, may decrease the incidence of FO23. In our study, 36 patients (27.6%) had AKI. Patients with FO ≥ 5% detected presented up to 71.4% (p < 0.001).
Age, weight, surgical complexity, and extracorporeal circulation time are associated with higher FO. Patients with FO ≥ 5% or a maximum FO peak show a higher OI and inotropic score and higher mortality. The presence of FO ≥ 5% increases days of mechanical ventilation and hospital stay.











 nueva página del texto (beta)
nueva página del texto (beta)