Introduction
Acne is a chronic inflammatory disease of the pilosebaceous unit with multifactorial etiology1. It is considered one of the most frequent diseases in adolescents and young adults, affecting 85% of individuals between 12 and 24 years of age and compromising 9.4% of the population worldwide2,3.
Besides those traditionally described, current evidence on the pathogenesis of acne shows that certain factors influence the development and exacerbation of lesions, such as genetics, stress, and, especially, diet4.
Historically, there has been controversy about the role of diet in the pathogenesis of acne. Early studies emerged at the beginning of the 20th century; however, the results were considered conflicting and inconclusive due to limitations such as inadequate sample size, lack of control groups, and blinding, among other factors5.
The study of the relationship between diet and acne gained interest until the 1960s and 1970s due to the myth of worsening acne caused by chocolate consumption. Thus, Fulton et al., in 1969, published a study showing that the consumption of cocoa solids-based foods was not associated with increased acne severity, but rather the intake of the other ingredients added to commercial chocolate bars6.
At the beginning of the 21st century, studies on the relationship between diet and acne reappeared. Notably, Smith et al. in 2007 compared two groups of patients with acne: the first group followed a diet rich in moderate/high glycemic index (GI) carbohydrates, and the second group followed a diet with a low glycemic load (GL). As a result, the second group showed a significant reduction in acne lesions and improved insulin sensitivity7. Furthermore, Tayel et al. in 2013 showed that the intake of a low GL diet in adolescents and young adults resulted in a significant improvement in acne severity8. Finally, Çerman et al. in 2016 demonstrated that a high GI and GL diet was directly related to acne, reaffirming studies conducted years earlier and establishing a starting point for future research and a potential target for treatments9.
In 2014, Burris et al. studied the relationship of GI and consumption of certain foods in patients with acne of different severity, finding that patients with moderate to severe acne reported higher GI in their diet and increased intake of milk, saturated fats, and trans fats. Moreover, 58.1% of participants perceived that diet aggravated or influenced their acne10. The same authors published in 2017 a study in which they evaluated the relationship between GI, GL, and biological factors associated with acne, and reported that acne patients consumed foods with higher GL, which increased insulin and insulin-like growth factor-1 (IGF-1) concentrations11.
Fabroccini et al., in 2016, conducted a study comparing two groups of patients with acne and an altered metabolic profile. One group received a hypocaloric diet and metformin, and the other did not. At the end of the follow-up, it was found that the patients who received treatment showed a significant improvement in acne severity, corroborating the relationship between insulin resistance and aggravation of the disease12.
Pathogenesis
The pathogenesis of acne consists of four main aspects: 1) increased androgens that stimulate keratinocyte proliferation, sebum production, and sebaceous gland growth; 2) abnormal keratinocyte proliferation leading to the formation of comedones; 3) inflammation of the pilosebaceous follicle, and 4) bacterial colonization by Cutibacterium acnes. However, in recent years, the diet has gained importance, and some biochemical markers modified by the diet directly influence the severity of acne11.
Biochemical markers
IGF-1
IGF-1 is a potent mitogen synthesized mainly in the liver that stimulates sebocyte growth and lipogenesis in the sebaceous glands9,13,14. It also induces androgen production in ovaries and testes and inhibits hepatic synthesis of sex hormone-binding globulin (SHBG) and IGF-binding proteins (IGFBP)-1 and IGFBP-3. SHBG is a glycoprotein that binds sex hormones (specifically testosterone and estradiol and has a high affinity for dihydrotestosterone), inhibiting their function and bioavailability. In addition, IGFBPs modulate the half-life of IGF and even modulate its interaction with its receptor. Therefore, inhibition of IGFBPs and SHBG increases the bioavailability of androgens and IGF-1, thereby increasing the severity of acne13,15,16 (Figure 1).
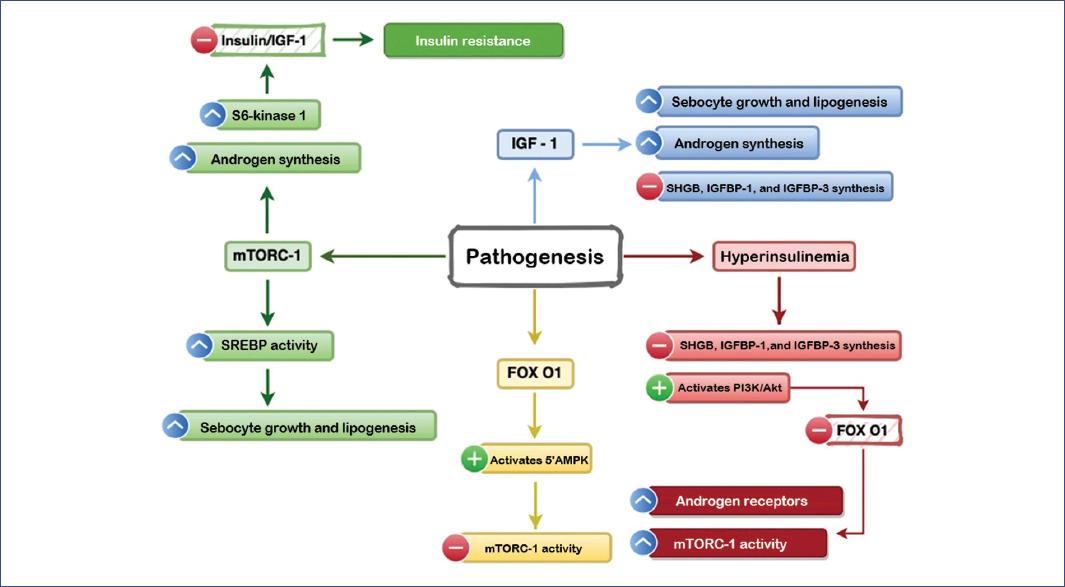
Figure 1 The actions of IGF-1 (insulin-like growth factor), FOX O1 (forkhead box O1), and mTORC-1 (nutrient-responsive mammalian target of rapamycin complex) markers in the pathogenesis of acne.5’AMPK, 5’-adenosine monophosphate-activated protein kinase; IGFBP, IGF-binding protein; SHBG, sex hormone-binding globulin; SREBP, sterol regulatory element-binding protein.
INSULIN, FOX O1, AND MTORC-1
Insulin response to glucose increases during puberty and adolescence, while insulin sensitivity decreases significantly. These changes are essential, as insulin can completely modify the androgen axis, which is necessary during this stage of sexual maturation. Insulin targets the liver, where it inhibits SHBG production17.
Moreover, hyperinsulinemia can promote the appearance of acne by two mechanisms: in the first, it inhibits hepatic production of IGFBP-1 and IGFBP-3 and increases androgen synthesis17; in the second, it activates the phosphatidylinositol-3-kinase/Akt pathway, which reduces FOX O1 (Forkhead box O1) protein expression at the nuclear level11,18. FOX O1 is a transcription factor that inhibits protein synthesis, cell growth, and lipid metabolism17,18. Under normal conditions, FOX O1 induces activation of the 5’ adenosine monophosphate-activated protein kinase (5’AMPK) pathway, which is a critical negative regulator of the nutrient-responsive mammalian target of rapamycin kinase (mTORC)-115,17.
FOX O1 depletion causes an increase in androgen receptor activity and mTORC-1 activity in the pilosebaceous unit11,12. mTORC-1 is a serine/threonine kinase that functions as a regulator of cell growth, proliferation, lipid synthesis, and protein transcription; it is sensitive to growth factors (insulin, IGF-1), energy levels (glucose, AMP/ATP ratio), and some amino acids (leucine)19,20.
Increasing androgen synthesis also increases androgen receptor activity, which inhibits the DEP domain of mTORC-1 (DEPTOR), activating mTORC-1. As a result, keratinocyte proliferation and differentiation are increased and lipid synthesis in the pilosebaceous unit20,21.
When mTORC-1 activity is increased, transcription of the sterol regulatory element-binding protein (SREBP) gene also increases. This protein regulates the synthesis of cholesterol, fatty acids, triglycerides, and phospholipids and stimulates lipogenesis and sebaceous gland enlargement9,22. Subsequently, C. acnes triacylglycerol lipase converts the triacylglycerols present in normal sebum into free fatty acids, such as palmitic acid, sapienic acid, and oleic acid, stimulating the formation of biofilms23.
Increased mTORC-1 activity and decreased FOX O1 also stimulate androgen and S6 kinase-1 secretion12,13. Furthermore, S6 kinase-1-mediated insulin receptor substrate-1 phosphorylation downregulates insulin/IGF-1 signaling and consequently induces insulin resistance (Figure 1)14,20.
GLYCEMIC INDEX AND GLYCEMIC LOAD
The glycemic index (GI) measures the effect of carbohydrates from a given food on postprandial glucose concentration. Food with a high GI is characterized by rapidly digested and absorbed carbohydrates that can significantly increase blood glucose and insulin concentrations17. Conversely, glycemic load (GL) considers the quality and quantity of carbohydrates consumed and, therefore, estimates the overall glycemic effect of a standard portion of a given food9,17.
High GI and GL induce hyperinsulinemia, thus stimulating increased concentrations of IGF-1 and androgens15,18, which can also activate the mTORC-1 receptor and SREBP, resulting in the amplification of pathways of acne pathogenesis17 (Figure 2).

Figure 2 EPA, n-3 eicosapentaenoic acid; FGF, fibroblast-derived growth factor; FOXO1, forkhead box O1 protein; GI, glycemic index; GL, glycemic load; IGF, insulin-like growth factor; IL, interleukin; LTB4, leukotriene B4; PDGF, platelet-derived growth factor; PGE2, prostaglandin E2; PUFA, polyunsaturated fatty acids; SREBP, sterol regulatory element binding protein; TGF, tumor growth factor; TLR, Toll-like receptor; mTORC, nutrient-responsive mammalian target of rapamycin complex.
ADIPONECTIN
Adiponectin, a hormone derived from adipocytes and produced mainly in subcutaneous fat, has anti-inflammatory, antioxidant, and anti-diabetic properties. It inhibits proinflammatory cytokines and induces anti-inflammatory cytokines, down-regulates the expression of Toll-like receptor (TLR)-2 ligands and receptors, and increases insulin sensitivity. Additionally, adiponectin inhibits mTORC-1 activity by activating 5’AMPK. GI, GL, body mass index, and obesity are inversely related to adiponectin concentrations2,9,17 (Figure 2).
Western diet
The Western diet is characterized by high GI foods, refined grains, red meat, milk and dairy products, egg protein, and saturated fats9,14,22.
This type of diet increases GL, insulin production, IGF-1, and leucine levels; in turn, upregulation of these factors decreases FOX O1 activity, thus losing the ability to inhibit androgen receptors and mTORC-1 activity. Moreover, these factors also stimulate basal keratinocytes to release interleukin-1 (IL-1) and other cytokeratins that result in hyperproliferation with hypercornification of the follicle wall, which is the precursor event for the formation of comedones14,22.
The Western diet is also high in linoleic acid, a peroxisome proliferator-activated receptor-gamma (PPARg) ligand that strongly stimulates lipogenesis in sebocytes and maturation of follicular keratinocytes14,22.
Hypotheses on the influence of diet on acne pathogenesis are supported by observing low incidence of acne in cultures with “paleolithic diets”—composed of minimally processed foods, vegetables, low amounts of carbohydrates, and no dairy or its derivatives18,22. Furthermore, these diets contain high levels of omega-3 and omega-6 polyunsaturated fatty acids (PUFAs), which are essential mediators of inflammation and positively impact acne24,25.
Regular fish intake has also been reported to reduce acne, as it contains high levels of n-3 eicosapentaenoic acid (EPA), which acts as a competitive inhibitor of the conversion of arachidonic acid (AA) into inflammatory mediators such as prostaglandin E2 (PGE2) and leukotriene B4 (LTB4), reducing acne-associated inflammation13,26.
In contrast, saturated fatty acids in the Western diet increase TLR2/IL-1B receptor expression, promoting TH17 cell differentiation and increasing IL-17A secretion. Increased IL-1B and IL-17A can be found in all acne lesions. IL-17A contributes to keratinocyte hyperproliferation and decreases their differentiation27,28.
Some studies have reported that low intake of vegetables and fruits can aggravate acne. In contrast, the Mediterranean diet—rich in vegetables, fruits, antioxidants, unsaturated fatty acids, and low GI foods—has a protective effect against developing this condition13,29,30 (Figure 2).
Food and beverages
MILK AND DAIRY PRODUCTS
Milk consumption increases the risk of acne and its severity. Milk is a complex fluid composed of several carbohydrates, proteins, and hormones21,31. In addition, dairy products contain high levels of branched-chain amino acids (BCAAs), such as leucine and palmitic acid, which increase insulin secretion14,21. Leucine also stimulates mTORC-1 and SREBP, increasing lipogenesis in the sebaceous glands14,20. Thus, elevated serum BCAA concentrations are related to oxidative stress and inflammation via mTORC-123,32.
Milk also contains and induces IGF-1, thus decreasing FOX O1 and increasing mTORC-1 activity31,33. Other studies have shown that milk consumption causes a disproportionate increase in insulin levels (despite having a low GI), producing an insulin response 3 to 6 times greater than its corresponding GI31.
Depending on the level of fat, cow’s milk is classified as whole milk (3.5%), low-fat milk (2%), and skim milk (fat-free), the latter being associated with higher plasma IGF-1 levels13,31. However, Ulvestad et al. demonstrated in 2016 a direct relationship between acne and high milk consumption regardless of milk fat content, which would reveal that the pro-acne effect of milk is associated more with its high content of hormones and bioactive molecules than with fat content33,34.
Milk contains whey protein, which is highly acnegenic22. Extracts of this protein contain six growth factors: tumor growth factor (TGF), IGF-1 and IGF-2, platelet-derived growth factor (PDGF), fibroblast growth factor-1, and FGF-2, all of which are potent inducers of glucose-dependent insulinotropic polypeptides that stimulate insulin secretion in pancreatic b cells13. Natural milk contains 1% whey protein, compared to 2% in processed milk. In addition, reduced-fat milk often has added whey protein to balance the caloric content22. Whey protein is also a dietary supplement popular among athletes and can cause moderate to severe acne14,20.
Also, milk comes from 75-90% of pregnant cows, which confers high progesterone, androstenedione, dehydroepiandrosterone (DHEA), and dihydrotestosterone (DHT) content. These hormones increase the expression of androgen receptors and, consequently, the activation of mTORC-113,21.
Finally, recent studies have shown that Western diet and milk intake reduce the activity of the transcription factor p53, implicated in the pathogenesis of acne and prostate cancer. In addition, epidemiological findings highlight a correlation between the onset of acne in late adolescence and an increased risk of prostate cancer13 (Figure 2).
ALCOHOL
A significantly higher frequency of alcohol consumption has been reported in patients with acne. Alcohol has also been shown to increase testosterone levels and the production of proinflammatory cytokines. In addition, it suppresses the immune system in the long term, allowing bacterial proliferation with alteration of the skin microbiome and exacerbation of acne; when excreted in sweat, it acts as a nutrient for C. acnes13,35.
TEA, COFFEE, AND CHOCOLATE
To date, there is no consistent data to suggest that tea, coffee, or chocolate can induce or aggravate acne. The factor that triggers acne is the sugar added to these beverages. Contrary to popular belief, studies show that the polyphenols in green tea have antimicrobial activities and can reduce sebum secretion, thus benefiting acne13,36.
Vongraviopap and Asawnonda, and other authors have reported that 99% of dark chocolate exacerbates acne due to its content of saturated fatty acids, sugar, and milk13,21,37.
Supplements
VITAMINS A, D, AND B12
Oral supplementation with vitamin A and D (1,25D3) has been related to their immunomodulatory capacity since the consumption of these vitamins inhibits the differentiation of Th17 cells, preventing the production of IL-17A. In addition, vitamin D can inhibit mTORC-1 activation and increase the production of cathelicidins against C. acnes. Findings have shown vitamin D deficiency in up to 48% of acne patients, demonstrating an inverse relationship between vitamin D concentrations and disease severity21.
Regarding vitamin B12 (hydroxocobalamin), Kang et al. conducted a longitudinal study in 2017. They found that the biosynthesis pathway of this vitamin was negatively regulated in acne patients and healthy subjects receiving vitamin B12 supplementation. In healthy skin, when the levels of vitamin B12 are normal, the biosynthesis pathway of this molecule in C. acnes is adequate, and porphyrin biosynthesis is low38. As vitamin B12 levels in the host increase, transcriptional changes are induced in C. acnes, which diverts the metabolic flux of 2-oxoglutarate and L-glutamate to porphyrin biosynthesis and decreases vitamin B12 biosynthesis, demonstrating an inverse relationship37,39.
Excess porphyrins in the pilosebaceous follicle interact with molecular oxygen, generating free radicals that damage adjacent keratinocytes and stimulating the production of inflammatory mediators; thus, inducing an inflammatory response that subsequently results in the development of acne37,38.
Karadag et al. in 2011 and Johnson et al. in 2016 reported that vitamin B12 serum levels and porphyrins in the pilosebaceous unit decrease significantly after acne treatment40,41 (Figure 2).
ZINC
This micronutrient has demonstrated a bacteriostatic effect against C. acnes by inhibiting chemotaxis and decreasing the production of proinflammatory cytokines. It has been shown that acne patients have lower serum zinc levels and that zinc supplementation would reduce the inflammatory lesion count without significantly increasing the incidence of treatment-associated adverse effects42,43.
Skin-gut axis
The skin-gut axis concept proposes a relationship between alterations of the gastrointestinal microbiome with increased intestinal permeability, systemic inflammation, and acne onset. The gut microbiome influences oxidative stress, glycemic control, and adipose tissue metabolism. The literature estimates that 40% of acne patients have hypochlorhydria, which can cause migration of bacteria from the colon to the small intestine, leading to alterations of the microbiome and colonic bacterial overgrowth, causing direct damage to the epithelium, systemic inflammation, and possible cutaneous manifestations22.
Oral supplementation with probiotics (lactobacilli, bifidobacteria, and enterococci) has been associated with clinical improvement of acne, mediated by the production of antibacterial proteins and bacteriocin-like substances with immunomodulatory effect on keratinocytes, as well as by the reduction of proinflammatory cytokines and induction of CD8 cell recruitment, thus regulating the intestinal microbiome. Studies to date, although scarce, have shown that administration of Lactobacillus rhamnosus GG for 12 weeks significantly improved acne and that treated patients had lower IGF-1 expression and higher FOX O1 expression in skin biopsies, which has led to consider oral administration of probiotics as an adjuvant in the management of acne21,44-46.
Lifestyle
Modern lifestyles that include passive leisure, such as watching TV, playing video games, and working for hours in front of the computer, can lead to uncontrolled food intake, especially hypercaloric, high GI, or GL foods13. This type of diet leads to obesity and high body mass index, which have been linked to acne47.
Treatment
Patients should be asked about their eating habits, lifestyle, and family history of acne or eating disorders during the first medical visit to verify whether nutrition can influence acne. If deemed necessary, body mass index should be calculated to verify if the patient is overweight or obese, which would imply an increased risk of acne13.
Several randomized clinical trials demonstrate that low GI/GL diets can decrease acne severity25. Jung et al. conducted a randomized, double-blind trial in 2014, in which they showed improvement of inflammatory and non-inflammatory lesions by dietary supplementation of omega-3 or omega-6 for at least 10 weeks48. Omega-3 is found naturally in fish and seafood, and omega-6 is found naturally in sunflower, corn, and safflower oil21.
If the problem were the type of diet, patients should be advised to change their eating habits or even request the support of a nutritionist13. A review article reported the usefulness of metformin as an adjunctive treatment for acne therapy: its use significantly reduced the number of lesions, especially inflammatory lesions, with minimal side effects (diarrhea and flatulence)25. The usefulness of metformin is due to its activation of the 5’AMPK pathway, which is an indirect inhibitor of mTORC-115. Therefore, using metformin combined with a low-GL diet can be considered in patients who do not respond to treatment or present a rapid relapse13,25. The patient should also be reminded of the importance of a balanced diet: dairy products, insulinotropic cereals, fatty diets, fast food, and other foods with high GI should be reduced as much as possible. Instead, the patient should be encouraged to opt for antioxidants such as fruits, vegetables, and omega-3, change lifestyle, and increase physical activity49.
These measures act in conjunction with the known acne treatment, depending on its severity and each patient’s unique conditions: anti-androgenic agents, topical or systemic retinoids, topical or systemic antibiotics, and keratolytic agents15.
Understanding the multifactorial etiology of acne is key to providing a comprehensive approach to the patient. It is necessary to update the training and knowledge of dermatologists continuously, as new factors related to the severity and evolution of this disease are discovered every day. By knowing the primary molecular markers altered by diet and which are somehow involved in the pathogenesis of acne, a specialized treatment with behavioral education and individual counseling for therapeutic choice can be achieved.











 nueva página del texto (beta)
nueva página del texto (beta)


