Introduction
Internationally, cerebral aneurysms in pediatrics represent < 4% of all aneurysms1,2, and their rupture has a mortality rate of 10-23%. They have been associated with infections, traumatic brain injury (TBI), sickle cell anemia, cardiovascular diseases, autoimmune diseases, immunodeficiencies, connective tissue diseases, dysmorphic syndromes, and family history of aneurysms. The clinical presentation is due to subarachnoid and intraparenchymal hemorrhage and the mass effect caused by the size of the aneurysm, which can produce severe headaches, seizures, disorientation, motor-sensory deficit, and even death. Diagnosis is made by cranial computed tomography angiography (CTA), cerebral magnetic resonance angiography (MRA), and, ideally, with four-vessel cerebral digital subtraction angiography (DSA)3,4.
Aneurysms should be considered as one of the differential diagnoses in patients with the described clinical manifestations and associated pathologies for prompt diagnosis and treatment.
Clinical case
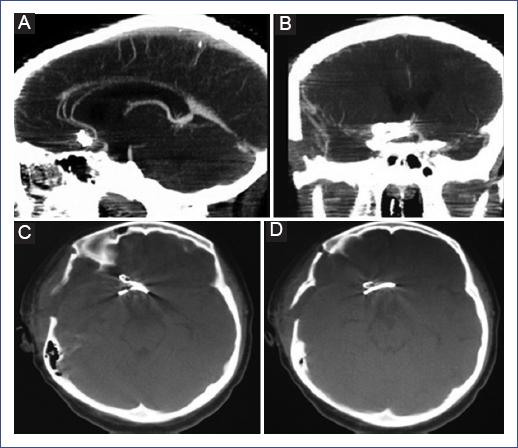
We describe the case of a 12-year-old female patient who presented with a sudden intense headache while at home and without exertion. She was initially managed with oral paracetamol at home, and after 72 hours, she developed generalized tonic-clonic seizures and was transferred to the hospital, where she was stabilized with antiepileptics and analgesics. She immediately underwent a simple cranial tomography, which showed an intraparenchymal hemorrhage within the right frontal lobe and a subarachnoid hemorrhage in the interhemispheric fissure. The study was complemented with a CTA, showing a 12 mm bilobed saccular aneurysm in the anterior communicating artery, so she was evaluated by the pediatric neurosurgeon (Figure 1).

Figure 1 A-C: Axial section of the simple cranial tomography showing a hemorrhage in the medial side of the right frontal lobe. D-F: Axial, coronal, and sagittal tomography-angiography showing bilobed aneurysm of the anterior communicating artery.
The patient was awake, with a tendency to somnolence, reactive isochoric pupils (3 mm), without cranial nerve involvement, left body hemiparesis with strength 4/5. She presented the following scores: on the Glasgow Coma Scale, 12 points (O3, V4, M5); on the World Federation of Neurologic Surgeons scale (WFNS), grade IV; on the Hunt and Hess scale, grade 3, and on the Fisher scale, grade 4 (by tomography). Management was decided in the Pediatric Intensive Care Unit (PICU) to ensure stability, and clipping of the aneurysm was performed by right pterional craniotomy two weeks after the event (Figure 2). The patient was extubated 72 hours after the procedure and subsequently returned to the ward for continued management.
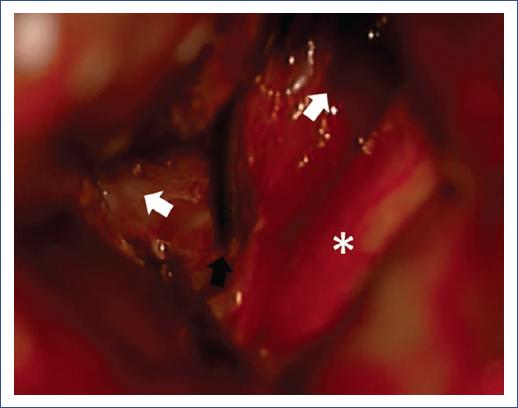
Figure 2 Intraoperative image showing the bilobed suprachiasmatic aneurysm. The optic chiasm (asterisk), the transient clip (black arrow), and the lobes of the aneurysm with anterior and superior projection (white arrows) are observed.
On day 7, a postoperative CTA was performed, which showed adequate clipping and exclusion of the aneurysm (Figure 3). The patient was evaluated by Rheumatology, Cardiology, Neurology, Infectology, and Genetics, performing whole body CTA, echocardiography, bilateral renal ultrasound, and immunological tests. However, it was not possible to identify the etiology of the aneurysm. She was discharged on postoperative day 8 with an antiepileptic drug and resolution of symptoms. She is currently asymptomatic with outpatient follow-up.
Discussion
Aneurysms are classified according to their size and morphology and can be single or multiple in 10% of cases. By size, they are small (< 5 mm), large (6-24 mm), or giant (> 25 mm). Morphologically, they are saccular and fusiform or dissecting. Their location has a predilection for the anterior circulation in 75% of cases, with the internal carotid artery, middle cerebral artery, and anterior cerebral artery being the most frequent locations, and the posterior circulation in 25%, most commonly in the basilar artery. The location and size are similar in pediatric and adult patients1,5.
In pediatric patients, aneurysm etiology is identified in < 50% of the cases; 5-10% are associated with TBI, 15% with infections, and 50% with vascular dissection. TBI-associated aneurysms cause a lesion of the three vascular layers, producing pseudoaneurysms and true aneurysms. Those related to infections (generally by Staphylococcus aureus, Streptococcus viridans, and other Gram-negative microorganisms) show vasculitis and consequent weakening of the vascular wall.
Dissecting aneurysms are caused by endothelial injury and transmural vascular dissection that weakens the muscular and adventitial layer. They are associated with medical-genetic diseases (polycystic kidney disease, aortic coarctation, aortic valve stenosis, moyamoya disease, sickle cell anemia, Kawasaki syndrome, Takayasu disease, human immunodeficiency virus (HIV), tuberous sclerosis, neurofibromatosis type 1, Ehler-Danlos syndrome, and Marfan syndrome)3,5.
In adults, aneurysms develop near a vascular bifurcation point, where the endothelium is damaged, there is a thinning or absence of the muscular layer, and, frequently, there is fibrinoid material in the adventitial layer. These lesions are favored by alcohol abuse, smoking, aging, systemic arterial hypertension, and several medical (aortic aneurysm, aortic valve stenosis, aortic coarctation, Ehler-Danlos syndrome, fibromuscular dysplasia, among others) and genetic (hereditary hemorrhagic telangiectasia, intracranial arteriovenous malformation, Klinefelter syndrome, Marfan syndrome, neurofibromatosis, Noonan syndrome, pheochromocytoma, polycystic kidney disease, tuberous sclerosis, alpha-1 antitrypsin deficiency, and alpha-glucosidase deficiency) conditions. Alcoholism, smoking, and hypertension are rare conditions in the pediatric population2,6.
DSA is the gold standard for diagnosing aneurysms; however, it is increasingly common to use CTA and MRA, which have detected aneurysms larger than 3 mm in size1,2,7-9. DSA was not used in this study, as neurological endovascular therapy (NET) was not considered the first treatment line.
Ruptured cerebral aneurysms present high morbidity and mortality. Management by surgical clipping or NET should be performed once diagnosed. However, vasospasm and cerebral edema may limit such management. Therefore, the recommendations are to operate in the first 72 hours of the event or wait until cerebral edema decreases to favor microsurgical management and also in cases with poor neurological status. These recommendations are based on the adult population. In the case of multiple aneurysms, Burkhardt et al. proposed clipping the ruptured aneurysm first and then waiting up to 30 days to treat the rest, with no impact on the patient10. There is no consensus on the timing of surgery in the pediatric population. Unlike adults, in whom vasospasm is the leading cause of death, vasospasm is tolerated in children due to leptomeningeal circulation and higher cerebral blood flow, allowing more time to reduce cerebral edema and facilitate exposure of the aneurysm for clipping2,3,6,11.
Aryan et al. conducted a study of 50 patients with aneurysms, in which clipping was performed in 78.8%, all in the anterior circulation, and NET was used in 21.2% located in the posterior circulation12. Additionally, Sanai et al. reported similar results with both techniques in 32 patients: clipping was performed in 40% and NET in 60%, but they found residual aneurysm or recurrence in 19% of the NET group and no recurrence in the clipping group13.
Lasjaunias et al. conducted a study in 59 patients with aneurysms, in which they embolized (68.8%), clipped (21.9%), or used combined treatment in 9.3% of cases. These authors observed complete exclusion of the aneurysm in patients who underwent clipping and recurrence of 8.3% in those who underwent embolization14. Furthermore, in a study of 22 patients, Proust et al. performed clipping in 77.3%, NET in 18.2%, and combined therapy in 4.5%; in the patients in whom clipping was performed, the aneurysms were located in the anterior circulation15. In a series of 22 patients with aneurysms, 77% located in the anterior circulation and 23% in the posterior circulation, Slator et al. embolized 80% and performed clipping in 20%, without finding statistically significant differences between the treatments used16. In another case series of 6 patients < 1 year of age with giant aneurysms, Ren Y et al. recommended clipping since it allows draining the intraparenchymal hematoma, excluding the aneurysm and removing the mass effect, a situation not offered by NET. However, they recognize that NET produces less bleeding than clipping17.
Garg et al. treated 62 patients with aneurysms: 51.6% underwent clipping, 30.6% underwent NET, 6.4% underwent another type of treatment (bypass or ligation of the main aneurysm artery), and 9.6% underwent conservative management; 1.6% died before surgery. Of the aneurysms, 3.2% were of infectious origin, and another 3.2% were post-traumatic. The authors found no differences between the clipping group and the NET group, and patients did not have recurrence either; they mentioned that the treatment decision was influenced by its cost since clipping is less expensive than NET18. Requejo et al. treated 17 patients with aneurysms: 23.5% underwent clipping, 64.7% underwent NET, 5.8% were managed conservatively, and the other 5.8% died before surgical management. The authors reported similar results with NET and clipping19. Of 10 patients with aneurysms, Thioub et al. performed clipping in 7 and NET in one. One of the patients died during anesthetic induction, and another did not accept surgical management. Also, one aneurysm was of traumatic origin and four with possible infectious origin. The authors recommend clipping when socioeconomic conditions are unfavorable20. Ilovar et al. treated eight patients with cerebral aneurysms: one underwent clipping, four underwent NET, and three were managed conservatively (two incidental and one symptomatic), concluding that endovascular and microsurgical management are similar21.
In two international trials, Molyneux et al.22,23 demonstrated that clipping offers more prolonged survival and a lower risk of rebleeding than NET in managing cerebral aneurysms.
Four cases of aneurysms in pediatric patients have been reported in Mexico: Mercado et al. reported the case of a 14-year-old male patient with a posterior communicating artery aneurysm and aortic coarctation resolved with NET24. Martinez-Longoria et al. reported the second case: a 12-year-old female patient with HIV and a fusiform aneurysm of the right internal carotid artery associated with vasculitis. The patient was managed conservatively and presented remission of the aneurysm at 6 months25. Palomera Gómez et al. reported the case of a 10-year-old female patient with a large aneurysm of the right carotid apex26. Finally, Escobar-de la Garma et al. reported the case of an 11-year-old male patient with a small aneurysm of the left internal carotid artery bifurcation27. These last two cases were resolved with clipping of the aneurysm.
In conclusion, pediatric cerebral aneurysms differ from their adult counterparts mainly in their etiology and evolution. Although aneurysm clipping and NET have shown similar results, more recommendations are still needed to choose the best option in children. Due to a longer life expectancy, pediatric patients should be offered the best therapeutic option.











 nueva página del texto (beta)
nueva página del texto (beta)