Introduction
The present work reviews the results from a long-lasting research project on prenatal undernutrition and brain development. For a long time, biomedical researchers have been interested in early developmental negative influences on the brain of mammals, particularly humans, due to secondary prenatal stress or restricted nutrition, acting together or separately to influence different brain processes during the early development1. In addition, the effect of protein-calorie undernutrition has been thought to profoundly affect human brain development2,3, given its general appearance worldwide, particularly in regions that continue to confirm the “Malthusian Principle of Population Growth,” related to food production inequality4.
Early malnutrition affects some general functions of the human brain, but these observations have always been challenging to interpret, particularly regarding the basic mechanisms underlying these effects5, such as protein content, number of brain cells, and lipid metabolism. Also, other general unspecific biochemical parameters have been studied, for example, specific brain functions5-11.
More recently, Morgane et al.12 reported functional alterations of the hippocampus in early protein-deficient undernourished rats, related explicitly to stressful early conditions. Barros et al. (2006)13 reported a decrease in dendritic development in early-life stressed rats13. Interestingly, Monk et al. (2013)1 reported a decrease in DNA, RNA, and mRNA coding for neural and glial structural proteins after maternal prenatal distress in animal models, decreasing neurotransmitter peptides, independently from the nutritional condition1.
Serotonin and its function in the brain
During intrauterine life, increased serotonin production in the fetal brain due to nutritional stress or after the gene knock-out (KO) of the enzyme in charge of serotonin inactivation (MAOA) produces structural changes and delayed development in the somatosensory cortex S114-17.
Serotonin (5-HT, 5-hydroxytryptamine) is an amine produced by a group of brainstem neurons. Axons from these neurons innervate various important areas of the central nervous system (CNS)18-22. 5-HT has been implicated in regulating brain development before it assumes its role as a neurotransmitter and neuromodulator in the mature brain23-29. During the fetal period, 5-HT is involved in neuronal growth and differentiation processes, axogenesis25,27,28, maturation of target neurons26, the final expression of specific 5-HT receptors30, and modulation of its synthesis31. As a neurotransmitter, 5-HT controls numerous physiologic functions (food intake, temporal control, sleep patterns, nociception)32-36; also, in some psychiatric disorders such as anxiety and depression37 in the adult brain. Serotonin functions in the brain are mediated by activating at least 15 different types of receptors that belong to the G protein-coupled superfamily (except for the 5-HT3 subtype)23,31,38,39.
One relevant fact is that 5-HT is synthesized from an essential nutrient, L-tryptophan (L-Trp), its metabolic precursor. There are two known fractions of plasma L-Trp, one bound to albumin and one free (FFT, free fraction of L-Trp)40. FFT crosses the blood-brain barrier (BBB) to the brain41,42, where it is hydroxylated by the action of the tryptophan-5-hydroxylase (TPH), decarboxylated, and transformed into 5-HT43.
Nutritional stress and intrauterine growth restriction
As our group has long pointed out, pre-, peri-, and postnatal undernourishment also cause alterations in specific brain systems during the prenatal to postnatal developmental periods, which is the case of the serotonin neurotransmission system. These alterations activate the brain's serotonergic biosynthetic system and metabolic processes, with an elevation of the FFT, 5HT metabolic precursor.
Furthermore, it has been demonstrated that plasma albumin changes its ability to bind L-Trp44-52. FFT crosses from plasma to the brain through the BBB and is taken up by brainstem serotonergic neurons to activate serotonin synthesis41,43,46,49,51,53,54. FFT significantly increases in the plasma and the brain of rats with intrauterine growth restriction (IUGR), reaching levels higher than those of normal controls, showing that the activity of TPH, the limiting enzyme system in the brain's serotonin biosynthetic pathway55-58, is upregulated in IUGR brains exhibiting significant changes in its kinetics: increased affinity (lower Km) for its substrate, no changes in the Vmax, and increased activity under phosphorylation conditions. These changes were considered responsible for the chronically increased serotonin synthesis in the brain of IUGR animals secondary to undernourishment59,60.
Two TPH isoforms have been described, TPH1 and TPH261. According to the authors who described these TPH isoforms, TPH1 is localized only in peripheral tissues, while TPH2 is present, supposedly only in the CNS62-66. Thus, alterations produced by IUGR induce an activation of the brain's serotonergic biosynthetic system and its metabolic correlates, with an elevation of the FFT and the plasma albumin's ability to bind L-Trp52. These findings suggest that chronic elevation of 5-HT synthesis in the brain of IUGR rats might be due to a significantly higher amount of TPH1 isoform above that of TPH2. This relation is possibly due to changes in the molecular regulatory mechanisms of enzyme expression and regulation of its activity, secondary to early nutritional stress during fetal life, with possible mediation of the Pet-1 regulatory molecular system67-69.
In contrast, when the undernourished offspring were subjected to a nutritional recovery (NR) regimen during the neonatal period, a satisfactory recovery was demonstrated in their growth curves and FFT and other plasma biochemical markers, which returned to control values70-74. Despite this impressive recovery, the whole TPH activity and TPH1 protein levels remained significantly elevated in the animal brainstems, while a significant increase in brain 5-HT levels and its functional activity also prevailed up to adulthood in IUGR rats70-74.
As an attempt to integrate our knowledge about the consequences of IUGR on the brain's neurochemistry and functions in rats, this review considered all published results, as well as the results of previous studies in human infants related to the capacity of another essential protein, plasma albumin, to bind to the precursor amino acid L-Trp, which appears to be a critical regulatory system of the brain's serotonin synthesis. In this manner, we attempted to provide more information on the neurometabolic mechanism implicated in the persistently increased synthesis of 5-HT in the brain of IUGR subjects.
Experimental models of intrauterine growth restriction
The methods considered in this review were taken from published studies44,45,48-50. However, it is worth mentioning that an experimental model of gestational protein-calorie deprivation and early ligation of one branch of the uterine artery in rats was used in an attempt to reproduce conditions of protein-calorie malnutrition and placental insufficiency present in humans. These two experimental procedures would mimic the clinical conditions in pregnant women giving birth to IUGR products. These two methods are complementary in their effects: one involves nutritional and endocrine maternal imbalance, while the other would exclude these variables44,45. In a previous study, we evaluated the tracing of thalamocortical fibers in the offspring of IUGR rats and controls on postnatal days 1, 3, and 5. We also evaluated the immunostaining pattern of the serotonin transporter (SERT) and the serotonin receptor 5-HT1B with the specific antibody for each molecule17,75.
All experimental procedures performed on animals followed the Care and Use of Experimental Animals guidelines published by the Mexican Ministry of Health (NOM-062-ZOO-1999; August 22, 2001). Additionally, the Research Ethics Committee of the National Scientific Research Committee of the Mexican Institute of Social Security (IMSS, for its Spanish acronym), Mexico City, Mexico, authorized all human and animal research protocols (registry numbers: 2005-785-078; 2006-3604-07; 2006-2605-08; 2007-3604-12; 2014-785-052).
Intrauterine growth restriction in newborns
All procedures in infant patients, diagnoses, and clinical care were closely followed by experienced specialists76-78. The general conditions of how this review was organized for the various studies conducted in human infants can be wholly reviewed in Hernandez et al.45,52 and Manjarrez et al.46,47,49,51.
For the human segment of the whole project, we include a representative summary of the procedure followed in a case-cohort study in 37 newborns during the first 3 months of postnatal life. At birth, two groups were formed; the first group (IUGR group) included 20 term newborns with the antecedent of IUGR, with bodyweight < 10th percentile of intrauterine growth curves79 and with a fetal growth ratio (FGR) of < 0.9080. The control group comprised 17 newborns with bodyweight between the 10th and the 90th percentiles and an FGR of > 0.90. Interestingly, at 30 days of age, nine infants of the IUGR group demonstrated a return to normal physical growth; subsequently, these infants formed the nutritionally recovered group (NR group) (Table 1). Free, bound, and total L-Trp were measured in blood micro-samples. These samples were freed of fatty acids and tested under “mole-to-mole” conditions of the sample components of the IUGR, NR, and control groups to assess the binding kinetics of L-Trp to albumin.
Table 1 Representative clinical data of infants of the various groups
| Controls | Intrauterine growth restriction | Nutritionally recovered | |
|---|---|---|---|
| Gestational age (weeks) | 39.5 ± 0.7 | 39.1 ± 1.20 | – |
| Ponderal index | 2.38 ± 0.26 | 2.10 ± 0.29€ | – |
| Fetal growth ratio | 98 ± 0.09 | 68 ± 0.05€; | – |
| Body weight (g) | |||
| 1 day | 3,165 ± 326.9 | 2,125 ± 234.5€ | – |
| 30 days | 4,093 ± 322.3 | 3,316 ± 332.3€ | 3,839 ± 193.9€ |
| 90 days | 5,703 ± 438.2 | 4,671 ± 387.1¥ | 5,571 ± 333.9€ |
| Body length (cm) | |||
| 1 day | 51.06 ± 1.0 | 46.63 ± 1.6€ | – |
| 30 days | 56.33 ± 2.9 | 51.06 ± 1.5€ | 54.00 ± 2.9€ |
| 90 days | 62.60 ± 2.4 | 58.38 ± 1.0€ | 61.86 ± 2.1€ |
| Body mass index | |||
| 1 day | 12.15 ± 1.13 | 9.90 ± 1.10 € | - |
| 30 days | 14.23 ± 1.23 | 12.46 ± 0.84 € | 13.89 ± 1.02 ¥ |
| 90 days | 15.36 ± 1.02 | 12.74 ± 1.30 ¥ | 15.28 ± 1.28¥ |
Data are expressed as mean values ± standard deviation of 17 controls, 20 intrauterine growth restriction, and nine nutritionally recovered infants.
Body weight (Treatment: SS = 11340, Df = 7, MS = 1610. Residual SS = 7320, Df = 76, MS = 96320). Body length (Treatment: SS = 2226, Df = 7, MS = 317.9.
Residual SS = 286.7, Df = 70, MS = 4.096). Body mass index (Treatment: SS = 292.5, Df = 7, MS = 4178. Residual SS = 101.6, Df = 79, MS = 1.286). Differences were determined by Mann-Whitney U, ANOVA and Tukey's multiple comparison tests.
¥p < 0.01,
€p < 0.00152.
SS, the sum of squares; MS, mean square; Df, degrees of freedom.
Serotonergic activity in the brain
In animal models, the variables analyzed generally used included the following in each one of the experimental groups: serotonergic activity, L-Trp, TPH whole activity, and 5-HT concentration expressed as mean and standard deviations (SD). Also, the number of TPH1 or TPH2-immunopositive neurons in each age group was determined in six 4 μm-thick sections, in an area of 83 μm2 with a 40X objective (Infinity 1-Lumenera camera equipped with a 10X objective, aided with an Olympus microscope). The relative densities of the bands observed in the dorsal raphe nucleus (DRN) (ages 1, 15, and 21 days) were determined. Afterward, the groups were compared by two-way ANOVA (nursing time 1, 15, and 21 days, and maternal nutritional status in control and undernourished groups), and post hoc analyses using the Tukey comparison test were conducted; p-values < 0.05 were considered statistically significant.
In general, these findings confirmed previous results, showing that malnourished offspring showed higher L-Trp concentration, 5-HT content, and TPH whole activity in the brainstem. In contrast, L-Trp concentration in the brain returned to normal values in the NR groups. Regardless of their physical and biochemical recovery, TPH whole activity remained significantly elevated, accompanied by an increased synthesis of 5-HT up to the end of lactation and even during adulthood (Table 2).
Table 2 Representative serotoninergic activity in the brainstem of rat pups of various groups
| Age (days) | L-Trp (mMol/g wet tissue) | TPH (whole activity) (nmol 5-HTP/mg protein/h) | 5-HT (nmol/g wet tissue) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | IUGR | NR | C | IUGR | NR | C | IUGR | NR | |
| 1 | 45.50 ± 0.50 | 58.70 ± 0.68* | 57.70 ± 0.68* | 0.217 ± 0.010 | 0.310 ± 0.030* | 0.308 ± 0.030* | 0.720 ± 0.070 | 1.185 ± 0.019* | 1.067 ± 0.010* |
| 15 | 12.70 ± 0.28 | 21.30 ± 0.21* | 13.60 ± 0.10 | 0.337 ± 0.050 | 0.510 ± 0.030* | 0.512 ± 0.030* | 1.670 ± 0.050 | 2.370 ± 0.031* | 2.400 ± 0.010* |
| 21 | 12.70 ± 0.28 | 22.30 ± 0.20* | 11.60 ± 0.20 | 0.376 ± 0.030 | 0.500 ± 0.010* | 0.517 ± 0.010* | 1.840 ± 0.040 | 2.750 ± 0.070* | 2.180 ± 0.060* |
Each point corresponds to mean values ± standard deviation of six experiments in duplicate.
Differences were determined by two-way ANOVA and post-hoc analysis conducted using the Tukey test.
*p < 0.05 (C vs IUGR; IUGR vs NR; C vs NR). Days = Days after birth70,74,87
C, controls; IUGR, in-utero undernourished; NR, nutritionally recovered; L-Trp, L-tryptophan; TPH, tryptophan-5-hydroxylase whole activity; 5-HT, 5-hydroxytryptamine.
The identification of TPH1 and TPH2 in the brainstem from the different groups was obtained by Western Blot (Figure 1A and 1B). The expression of TPH1 remained increased in the IUGR group from birth, and TPH2 expression predominated and subsequently showed a significant decrease during the lactation period in controls. Interestingly, TPH1 expression remained increased, while TPH2 returned to normal values in the NR group.
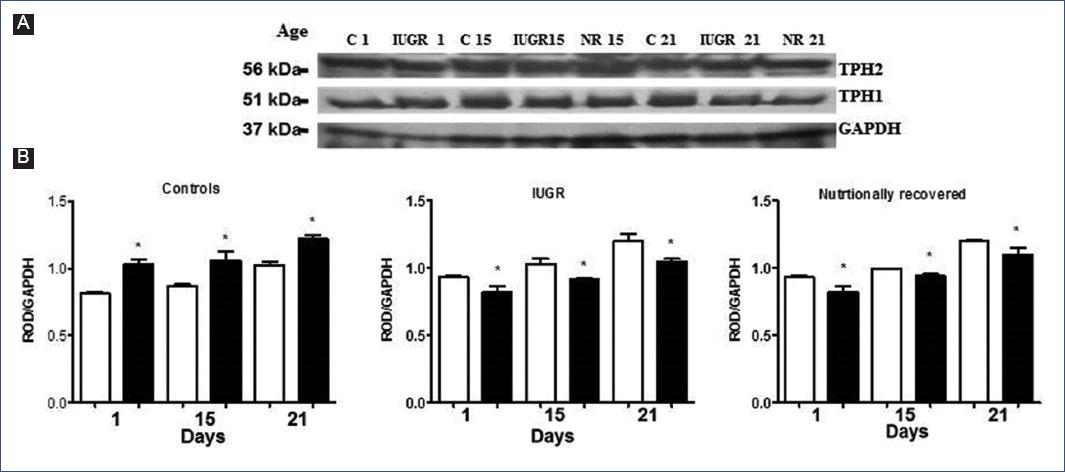
Figure 1 Identification of tryptophan-5-hydroxylases 1 and 2 (TPH1 and TPH2) in the brainstem of the offspring by electro-transference with specific antibodies to each isoform. A. Three bands were observed, one of 51 kDa (TPH1), another of 56 kDa (TPH2), and another of 37 kDa (GAPDH). B. Relative optical density (ROD) of each isoform; □: TPH1 y ■: TPH2. Each bar corresponds to mean values of ROD ± standard deviation of six experiments in duplicate of each isoform. *p < 0.05. (C vs IUGR; IUGR vs NR; C vs NR). A two-way ANOVA and post-hoc Tukey test analysis were conducted. C, controls; Days, days after birth87; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IUGR, intrauterine growth restriction; NR, nutritionally recovered.
A population of 5-HT neurons from the DRN was immunolabeled for TPH1 and another for TPH2 (Figure 2 and 3). Interestingly, the IUGR group showed fewer 5-HT immune-positive neurons (TPH1 and TPH2) than controls during the nursing period. It is essential to highlight that pups of the NR group exhibited a return from a decreased neuronal population to control values (Figure 4). Some morphological differences in the serotoninergic neurons between controls and the IUGR group, including the NR group, were having small neurons with little cytoplasm, small nuclei, and more space between them. In addition, immunolabeled serotoninergic neurons that express TPH1 increased in the IUGR and NR groups. Interestingly, the opposite was noted in neurons that expressed TPH2, in which the immunolabeling was lower than controls.
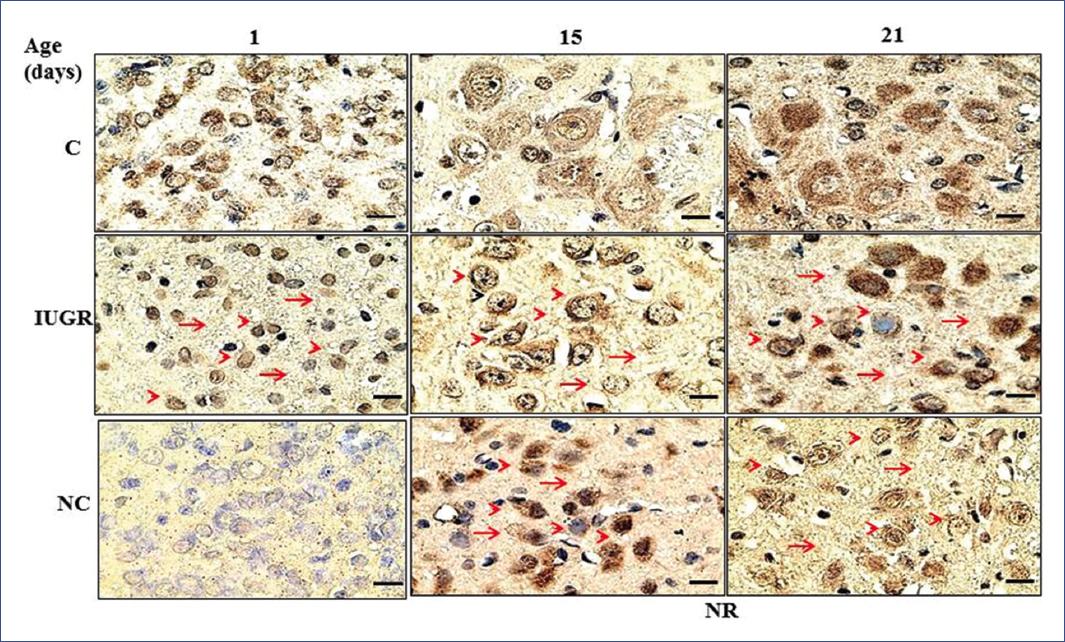
Figure 2 Coronal sections at the level of the DRN show immunoreactive neurons to tryptophan-5-hydroxylase 1. Sections were incubated with enzyme-linked monoclonal antibodies (1:1000), and immunoreactivity was detected with peroxidase-conjugated secondary antibodies and revealed with 3,3-diaminobenzidine on days 1, 15, and 21 after birth87. Scale bar in each panel = 100 X, 4 µm. Arrowhead = small neurons with little cytoplasm and small nuclei. Arrow = more space between neurons. C, controls; DRN, dorsal raphe nucleus; IUGR, intrauterine growth restriction; NC, negative control; NR, nutritionally recovered.
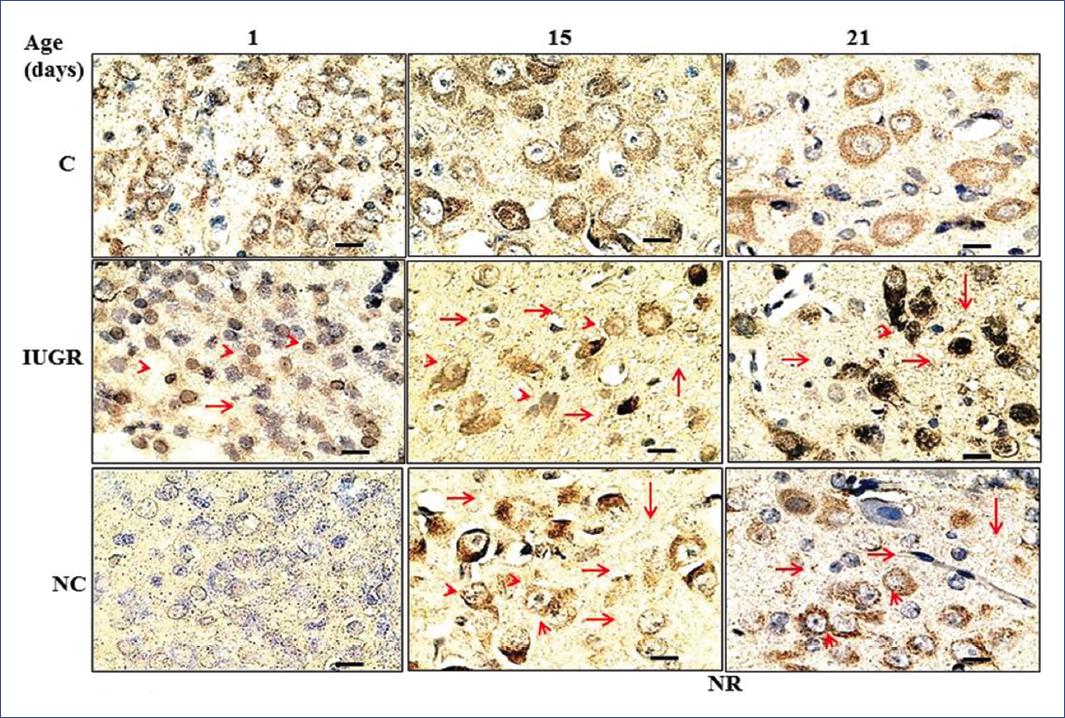
Figure 3 Coronal sections at the level of the DRN show immunoreactive neurons to tryptophan-5-hydroxylase 2. Sections were incubated with enzyme-linked monoclonal antibodies (1:1000), and immunoreactivity was detected with peroxidase-conjugated secondary antibodies and revealed with 3,3-diaminobenzidine on days 1, 15, and 21 after birth. Scale bar in each panel = 100 X, 4 µm. Arrowhead = small neurons with little cytoplasm and small nuclei. Arrow = more space between neurons87. C, controls; DRN, dorsal raphe nucleus; IUGR: intrauterine growth restriction; NC, negative control; NR, nutritionally recovered.
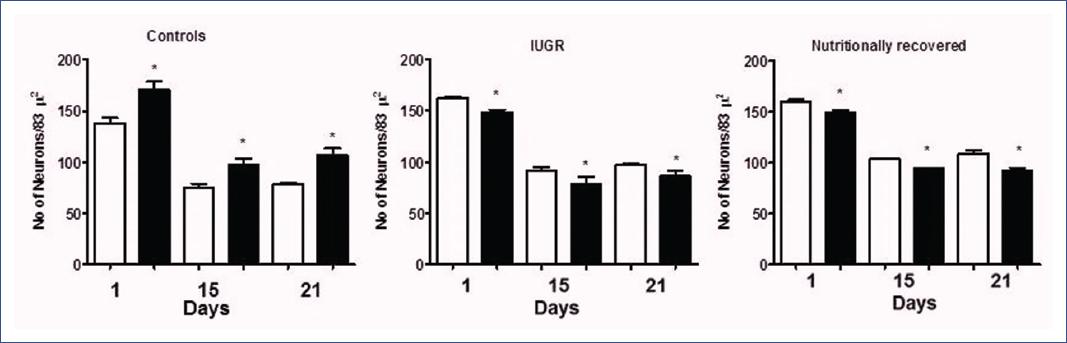
Figure 4 Tryptophan-5-hydroxylase-immunoreactive neurons in the DRN of the offspring. Each bar corresponds to the mean ± standard deviation of six pups of the groups. □TPH 1 and ■TPH 2. *p < 0.05. (C vs IUGR; IUGR vs NR; C vs NR). A two-way ANOVA and post-hoc Tukey test analysis were conducted. C, controls; Days, days after birth87; DRN, dorsal raphe nucleus; IUGR, intrauterine growth restriction; NR, nutritionally recovered.
The study in human infants confirmed that IUGR newborns exhibited a delay in physical growth. Remarkably, when they were fed only with maternal milk from birth, they demonstrated an appropriate recovering that allowed them to reach the growth rate of controls during the nursing period52. Also, IUGR infants showed significantly lower plasma albumin levels at birth, restored after 30 days of nutritional recovery. Furthermore, the FFT was significantly elevated in IUGR 3-month-old infants but returned to normal values on day 30 of postnatal life in the NR group. Another remarkable finding was that IUGR infants showed the following kinetic constants of albumin-L-Trp binding: high KD and low Bmax, both remaining unchanged up to postnatal day 90, even after NR52.
Discussion
We reviewed data derived from a long-lasting project confirming that undernourishment-related IUGR induces essential changes in the brain's serotonergic-metabolic pathway during early development and that this pathway is overactivated with a concomitant increase in 5-HT levels and functions.
In the related literature, data describes the importance of early alterations on the brain's serotonergic system in laboratory animals. Haydon et al. (1984)25,81 reported a strong effect on in-situ alterations induced by 5-HT excess. These 5-HT levels altered the axonal growth cone in cultured neurons, significantly inhibiting their growth, hence altering their developmental pattern. Also, early inhibition of the activity of the limiting enzymes by PCPA (P-chlorophenyl alanine) altered the maturation pattern of neurons innervated by 5-HT in different brain regions82. Fillion et al. observed that the early chemical lesion of serotonergic neurons by the intraventricular administration of 5,7-dihydroxytryptamine at birth increased the final number of 5-HT binding sites in rats developing brain30. Also, we observed that extra doses of L-Trp to gestational rats increased TPH whole activity, not only in the mother's brain but also in the fetal brain and serotonin synthesis83. Other authors have also reported a harmful effect of increasing serotonin levels in the brain during postnatal development84. Concerning prenatal brain differentiation, essential participation of 5-HT has been well known for some time in several aspects of this function26. For instance, we observed essential functions of 5-HT in the axonal growth cone particles (AGCP) isolated from rat fetal brain of 17 days of gestation27. These organelles are mainly responsible for axogenesis and synaptogenesis, among other functions. 5-HT produces serotonin uptake in a Na+-dependent manner, which is subsequently released in a K+ and Ca++-dependent-manner in the AGCP27,28, supporting the functional role of serotonin in brain neurodifferentiation.
Based on the experimental data published by our group, it is possible to state that fetal neurons have all the necessary elements to produce and use serotonin as follows: a) a regulatory plasma mechanism to obtain the precursor amino acid through FFT kinetic binding to plasma albumin, therefore regulating the amino-acid precursor availability52; b) a complete set of molecules to synthesize the neurotransmitter to take it up and release it physiologically; c) a set of specific high-affinity receptors, also identified in axonal growth cones85; and d) a molecular signaling cascade that transduces the 5-HT message to the target cells86. All these data illustrate how 5-HT works within the fetal brain (Figure 5).
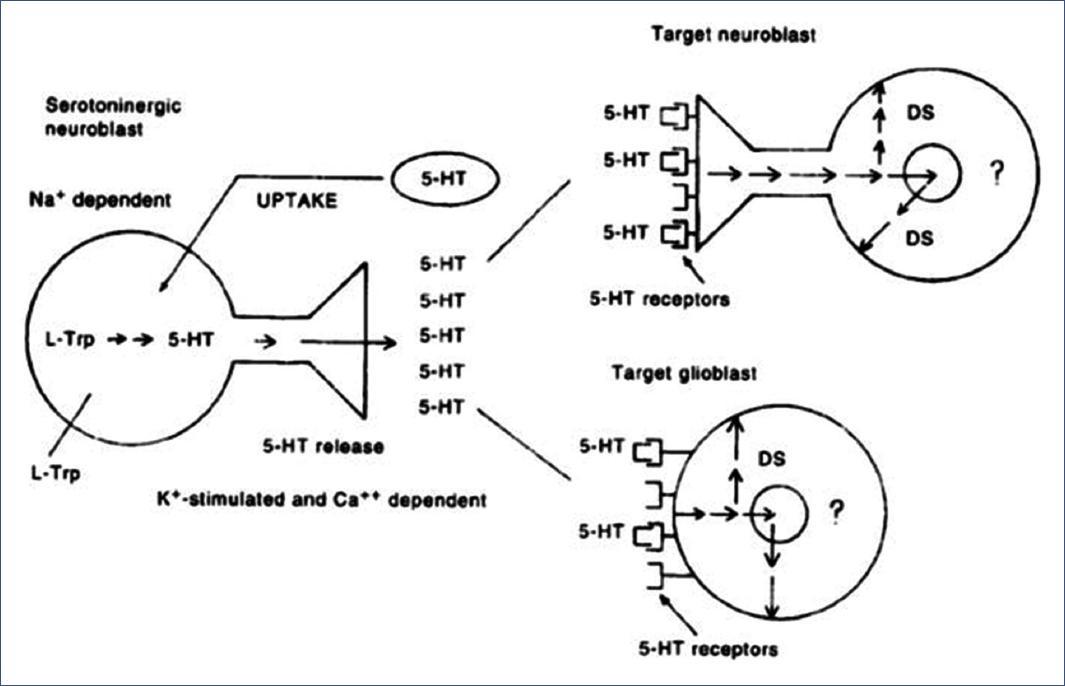
Figure 5 Proposed model of the physiological role of 5-HT in the fetal brain. Serotoninergic neuroblasts (left) synthesize and release 5-HT in a K+ and Ca2+ dependent way. Target neuroblasts (upper right) or glioblasts (lower right) would have 5-HT recognizing sites of a specific receptor that receive a differentiation signal whose sequence is unknown (?). 5-HT could be inactivated by a reuptake system dependent on Na+ and fluoxetine. DS, differentiation signal; 5-HT, 5-hydroxytryptamine65.
Unexpectedly, the elevated enzymatic activity in IUGR brains appeared to be mainly mediated through the activity of TPH1, which was significantly elevated. TPH2 was also present, but its identification by western blot was less evident than TPH1, although still higher than controls, as confirmed later87. Therefore, it appears that TPH1 activity in this area of the brain may play an essential role in the overactivation of the brain-serotonin pathway in both IUGR and normal brainstems, which could significantly alter the brain's morphogenetic processes, previously referred to in IUGR-malnourished rats44,45,50,59,60. By Western-blot analysis, both TPH isoforms (1 and 2) were expressed in serotonergic neurons in IUGR rats from birth61. In this context, it is important to mention that the intensity of the immunolabeling was significantly higher in neurons labeled with the specific anti-TPH1 monoclonal antibody. Interestingly, TPH2 neurons demonstrated a decreased immunopositive intensity at the end of the nursing period compared to the one exhibited by the TPH1 isoform61.
According to the literature, TPH1 expression in the brainstem of IUGR infants or normal eutrophic controls would not be expected because TPH2 predominates in the brain. Thus, the presence and activity of the TPH1-isoform in the brain of IUGR and controls require an acceptable explanation. This observation may be due to changes in the TPH regulatory expression exerted by the Pet-1 genetic regulatory system67-69. Alternatively, this metabolic modification consisting of the expression of TPH1 activity in the brain—higher than that of TPH2, which was supposed to be the only one active in the brain—could be secondary to hormonal changes due to the early profound stressful conditions exerted by the severe prenatal undernourishment.
Corticoids, other hormones, and nutritional changes may produce a de novo synthesis of enzymatic proteins. For example, it was reported that the genetic system of glucose-6-phosphate dehydrogenase responds to hormonal and dietary manipulations88. Thus, the enzymatic changes in the biosynthetic serotonin-brain pathway could also result from intense early nutritional stress during the prenatal period and subsequent effects on the genetic regulatory system at transcriptional and posttranscriptional levels. Therefore, it appears that activation of the involved gene's systems could be related to the unexpected presence of an enzyme that has been reported to function only in peripheral tissues62-66, inducing the expression of different enzymatic isoforms that are generally not present in the brain, as is the case of TPH1 in IUGR subjects. Alternatively, nutritional stress could activate a separate isozyme with an antigenicity like the one of TPH1. This phenomenon has been observed with other metabolic pathways, in which their limiting control rate can be reached in a tissue-specific manner by an adaptive regulatory process of a system of two genes, each one encoding a specific isozyme. Also, mRNA expression may be modified under fasting and re-feeding conditions, and dietary limitation of any essential amino acid may initiate a signaling cascade leading to an increase in the translation of a “master regulator” activating transcription factor, leading to the regulation of the DNA-RNA-protein pathway88,89. The functional overactivation of the serotonergic neuronal system in some brain areas, such as the sensory cerebral cortices, increases its function during the perinatal and nursing periods. This effect lasts up to adulthood in rat brains73,74 and up to 3 months in the postnatal life of infants who underwent IUGR (measured through indirect methods), affecting mainly the sensory cortices' normal responses to specific stimuli46,47,51,52.
Given that 5-HT has an important morphogenetic function in the process of neurogenesis15,26,86,90-93 and other differentiating events such as synaptogenesis27,28,81, we can also propose that disturbances may originate harmful effects exerted by IUGR nutritional stress in the prenatal serotonin functions. We also observed significant alterations not only in brain serotonin metabolism but also in the process of corticogenesis, particularly in thalamocortical connectivity during somatosensory (S1) cortex formation, adding evidence on how changes produced by IUGR stress on fetal serotonergic function are reflected in morphogenetic processes, with an abnormal morphologic S1 development16,17.
By non-invasive methods, we detected sensory cortex responses directly associated with serotonergic regulation in infants: N1/P2 auditory intensity-dependent recordings. These recordings provide information on the auditory cortex's responses to specific stimuli. In our experience47 and that of Hegerl et al.94,95, an inverse correlation was found between the amplitude and intensity of the N1/P2 component of the auditory evoked potential response that the cortical serotonergic activity could produce on the auditory cortex (A1), rich in 5-HT innervation, regulating its responses to specific stimuli. Consequently, we obtained interesting and significant information on the sensory cortex function indicators and plasma biochemical indicators of brain-serotonin metabolism in IUGR infants. These parameters were markedly abnormal in IUGR rats and IUGR infants born from mothers diagnosed with placental insufficiency and, thus, malnourished45-47,49.
When allowed to NR, early undernourished subjects showed a good catch-up to normality on their physical parameters. However, functional and biochemical changes were not recoverable despite NR and remained up to adulthood in the rat brain compared to controls70-74 and up to 3 months in infants52 (Table 1). This observation suggests the permanence of the alterations observed in the auditory cortex responses to specific stimuli, dramatically abnormal in infants who underwent prenatal stress with IUGR47 (Figure 6).
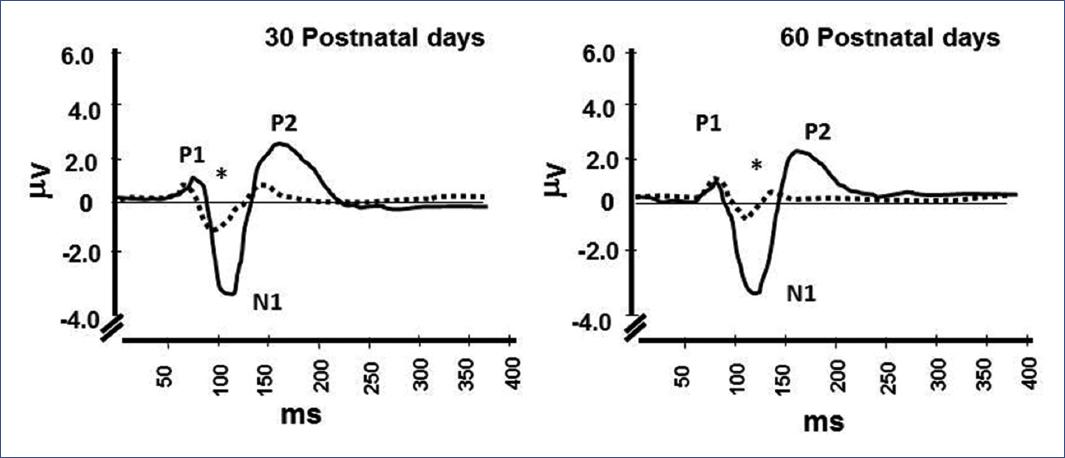
Figure 6 Auditory evoked potentials obtained from Cz reference electrode at a stimulation intensity of 60 dB in human controls (__) and infants with IUGR (---). Each recording represents the mean values from 12 and 13 infants in each group, respectively. *p< 0.001. Wilcoxon test, comparing N1/P2 component amplitude of IUGR with controls47. IUGR, intrauterine growth restriction
Another interesting question is why albumin capacity to bind L-Trp decreased significantly in IUGR patients' plasma. Plasma albumin from IUGR infants exhibited different binding properties than normal controls: a significantly higher KD and lower Bmax, indicating a lower affinity for the ligand (L-Trp) and a lower number of binding sites52,96. This kinetic behavior could explain higher FFT levels in the plasma of IUGR malnourished individuals, leading to an imbalance between plasma-free L-Trp and other neutral amino acids and the L-Trp/neutral amino acid ratio favoring the FFT45,49. In turn, high levels of FFT are transported across the BBB, activating the 5-HT biosynthetic pathway and, thus, reaching higher levels in the brainstem of IUGR individuals than controls. Unfortunately, there is no data on this binding phenomenon in humans, which possibly plays a relevant role in the serotonin metabolic pathway in the brain since FFT functions as a precursor for the synthesis of this brain neurotransmitter.
Authors who argue against albumin-L-Trp binding determinations have claimed that FFT is increased because plasma albumin concentration is decreased in malnourished individuals. However, in vitro binding experiments demonstrated that this is not the case because albumin concentration was the same, mole-to-mole, in plasma derived from eutrophic control infants and derived from IUGR subjects and free of fatty acids (FFA). Therefore, under these assay conditions, differences in L-Trp binding kinetics are not dependent on differences in plasma albumin concentration. These differences would be present if binding experiments were performed directly on whole plasma albumin samples with no prior FFA. Undoubtedly, if the kinetics of albumin binding to L-Trp were measured directly, not only would its concentration be variable, but other factors would probably interfere52. Therefore, our findings suggest a possible structural difference in human IUGR albumin that allows this protein to change its conformation97 in response to prenatal malnutrition stress. Thus, there is a different kinetic behavior for its binding to L-Trp in the plasma of human IUGR infants. In the context of serotonin metabolism in the brain, this phenomenon represents an essential peripheral regulatory mechanism for synthesizing this brain neurotransmitter, acting through the modulation of substrates for neurotransmitter synthesis. To our knowledge, this is the first time this has been described in newborns with IUGR and normal controls.
These findings support the notion that undernourishment distress during pre-, peri-, or postnatal development causes significant growth restriction and a possible change in TPH protein and plasma albumin structure, based on modifications in their kinetics and phosphorylation capacity52,59,60. Moreover, the decrease in TPH1-expressing serotonergic neurons coincides with a predominant enzyme expression compared with controls. A significant decrease of TPH2-immunoreactive neurons and a lower concentration of the enzyme suggest that stressful early conditions may induce epigenetic influences on the corresponding genes, as discussed previously, which shifts TPH expression toward TPH1 predominance, through a mechanism that, at present, is unclear, particularly concerning the presence of isoform 1 in controls.
Based on these results, it can be proposed that prenatal nutritional stress may significantly influence the brain's 5-HT biosynthetic pathway through mechanisms that may not necessarily be dependent on the genes encoding the enzymes but by epigenetic changes caused by intense nutritional stress and abnormal neurological changes induced by early undernourishment98-100. These remarkable enzymatic changes could be produced by modifications in the Pet-1 molecular apparatus, which appear to be essential in regulating enzyme expression in the serotonergic biosynthetic pathway from very early stages of brain development67-69,101. According to some authors, Pet-1 is also required to maintain TPH enzymes in the developing brain. Thus, significant alteration of the Pet-1-related molecular apparatus can be studied to gain further insight into the effects of early undernourishment on TPH enzymes activity in the developing brain. Another exciting possibility that would add information to this research project would be to establish the heritability of functional changes in tryptophan-5-hydroxylases and plasma albumin52,59,60,61 and their metabolic consequences on brain serotonin biosynthesis, which mainly affect the brain sensory function of IUGR infants. However, this phenomenon would be related to an epigenetic explanation.
In conclusion, our experimental data allowed extrapolation to human infants under similar pre-and perinatal developmental conditions. On this basis, the proposal of a psychopathological alteration of the brain-serotonin in these patients was supported, reinforced by the significant morphological, neurobiochemical, biochemical, and electrophysiological alterations affecting the serotonergic functions of the brain from the prenatal stage. These alterations could favor abnormal cognitive development or a predisposition to psychiatric abnormalities related to brain serotonergic function.
Experimental work continues in our group to obtain more information on this exciting and relevant topic.











 nueva página del texto (beta)
nueva página del texto (beta)


