Introduction
Acute appendicitis (AA) is the most common diagnosis requiring emergency surgery in the pediatric population1, with a prevalence of 69% in this age group2, of which 30-75% of cases progress to perforated appendicitis3.
According to some reports, the risk of progression from acute to perforated appendicitis in children is high4, with a mortality risk of ≈50%. This risk is higher than in the general population5,6, mainly due to the difficulty in diagnosing the complication, difficulties in physician-patient communication, and the absence of classic symptoms, resulting in delays in early treatment7,8.
The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are emerging as simple, low-cost markers that provide information on the action of two immune and inflammatory pathways9,10. These parameters have even been proposed as potential markers for predicting perforated appendicitis11-13 because they relate to innate immunity, which has an initial action on the inflammatory process and the long-term response of the immune system. Although studies have been performed in adult and elderly populations, there is uncertainty about using these markers in the pediatric population.
In countries with scarce health resources, such as Peru, these markers could help to identify patients at potential risk of perforated appendicitis in order to prioritize their admission to surgical wards, considering that surgical care times for appendicitis are usually long, which could lead to worse outcomes in this group of patients14,15. Therefore, this study aimed to evaluate the potential clinical utility of NLR and PLR as markers for the early diagnosis of acute perforated appendicitis in the pediatric population.
Methods
Study design
We conducted an observational, analytical cross-sectional study in which we performed a secondary analysis of a database of pediatric patients attended at the Regional Hospital of Ayacucho.
Study population, sample, and selection criteria
The study population consisted of all pediatric patients under 16 years of age at the time of diagnosis and who underwent appendectomy for acute appendicitis at the Regional Hospital of Ayacucho between January 2017 and December 2019.
For the present analysis, we obtained a non-random sample, in which we included all patients with an intraoperative diagnosis of acute appendicitis. We excluded those patients < 5 years of age (due to the essential physiological differences in lymphocyte counts below this age)16 who reported having pathologies that altered the NLR and PLR17, who had consumed any drug before admission, and who did not have any of the variables of interest reported.
Procedures
The necessary permissions were requested for access to the patient database and patient medical records. An author verified the data reliability by contrasting the information collected with the clinical history of each patient. Considering previous research, we also included variables that were not initially included but were necessary for the study and added them to the database11,18.
Outcome: perforated appendicitis
The diagnosis of non-perforated appendicitis was defined as congestive or catarrhal, phlegmonous or suppurative, gangrenous or necrotic appendicitis, and without macroscopic perforations or free fluid. In contrast, perforated appendicitis was defined by the presence of macroscopic perforations in the appendix and the presence of free intra-abdominal fluid. This variable was assessed based on the intraoperative report of each patient.
Independent variables
The leading independent variables were the NLR and PLR ratios. To obtain the NLR value, we divided the absolute number of neutrophils by the absolute number of lymphocytes, and for PLR, the absolute number of platelets by the absolute number of lymphocytes, as recommended elsewhere16. We decided to categorize the NLR and PLR variables considering the established cut-off points for the pediatric population proposed in published studies18. Therefore, categorical variables were obtained for both cases (NLR: ≤ 10.4 and > 10.4; PLR: ≤ 284 and >284).
Other variables were also evaluated, such as sex (male and female) and age (years), and clinical characteristics, such as fever, abdominal pain, nausea, vomiting, and diarrhea. Also, laboratory values such as leukocytes (≤ 15000 and > 15000 cells/mm3), neutrophils, lymphocytes, and their absolute values from the complete blood count were performed when the patient was admitted to the hospital. All the laboratory values were processed at the Regional Hospital of Ayacucho.
Statistical analysis
We used the Stata v.15 statistical software for data analysis. A descriptive analysis of the study population was performed using absolute and relative frequencies for categorical variables and central tendency and dispersion measures for numerical variables. The distribution of the variables was evaluated using quantiles plots.
The relationship between NLR and PLR and perforated appendicitis was calculated using logistic regression models, in which the odds ratio (OR) and their respective confidence intervals (95%CI) were obtained. To develop multiple models, we considered including known variables that could affect the primary relationship, such as age, sex, leukocytes >15 000 cells/mm3, as reported previously5,19. In addition, a sensitivity analysis was performed to compare the model created, including the confounding variables and the NLR/PLR vs. the model without the NLR/PLR variables, to determine the contribution of the primary variables NLR and PLR.
For the selected multiple regression models, receiver operating characteristic (ROC) curves were plotted, and their respective areas under the curve (AUC) were estimated. A cut-off point of 0.29 was chosen as the probability of having the outcome because it reports a better balance between its sensitivity, specificity, positive likelihood ratio (LR+), and negative likelihood ratio (LR-) values (Figure 1).
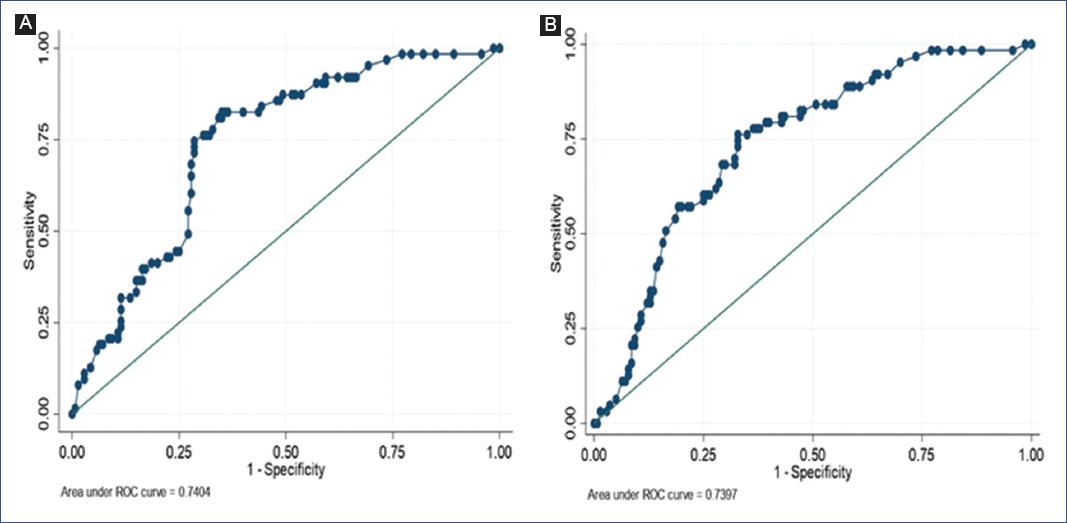
Figure 1 Receiver operating characteristic (ROC) curves of the predictive models. A. Predictive model for acute appendicitis and neutrophil-to-lymphocyte ratio, adjusted for age, sex, and leukocytes. B. Predictive model for acute appendicitis and platelet-to-lymphocyte ratio, adjusted for age, sex, and leukocytes.
Results
Of the 232 pediatric patients surgically intervened for AA at the Regional Hospital of Ayacucho between 2017 and 2019, we excluded 25 due to the absence of data on the variables under study, and four medical records were reported as missing. The final population study was of 203 patients with AA (mean age 10.9 ± 3.1), of which 31.0% presented perforated appendicitis.
Of this population, 32.5%, 99.5%, 53.7%, 16.3%, and 8.9% presented with fever, abdominal pain, vomiting, nausea, and diarrhea, respectively. Furthermore, within the laboratory values we obtained a mean of 10.7 ± 5.3 neutrophils (absolute value) and 1.5 ± 0.8 lymphocytes (absolute value) (Table 1). We observed that 37.9% showed values >15000 cells/mm3 of leukocytes, 38.9% values >10.4 in NLR, and 33.9% values >284 in PLR.
Table 1 Clinical characteristics of the patients with appendicitis (n = 203)
| Variable | Perforated appendicitis | |||
|---|---|---|---|---|
| n (%) | No 140 (68.97) | Yes 63 (31.03) | p-values | |
| Sociodemographic characteristics | ||||
| Sex | ||||
| Male | 110 (54.19) | 67 (60.91) | 43 (39.09) | 0.007 |
| Female | 93 (45.81) | 73 (78.49) | 20 (21.51) | |
| Age | 10.98 ± 3.10* | 11.39 ± 3.04* | 10.08 ± 3.07* | 0.005 |
| Clinical features | ||||
| Fever | ||||
| No | 137 (67.49) | 104 (75.91) | 33 (24.09) | 0.002 |
| Yes | 66 (32.51) | 36 (54.55) | 30 (45.45) | |
| Abdominal pain | ||||
| No | 1 (0.49) | 1 (100) | 0 (0) | 0.501 |
| Yes | 202 (99.51) | 139 (68.81) | 63 (31.19) | |
| Vomiting | ||||
| No | 4 (46.31) | 70 (74.47) | 24 (25.53) | 0.116 |
| Yes | 109 (53.69) | 70 (64.22) | 39 (35.78) | |
| Nausea | ||||
| No | 170 (83.74) | 112 (65.88) | 58 (34.12) | 0.031 |
| Yes | 33 (16.26) | 28 (84.85) | 5 (15.15) | |
| Diarrhea | ||||
| No | 185 (91.13) | 129 (69.73) | 56 (30.27) | 0.451 |
| Yes | 18 (8.87) | 11 (61.11) | 7 (38.89) | |
| Laboratory values | ||||
| Leukocytes | 12.97 ± 5.28* | 11.73 ± 5.05* | 15.73 ± 4.73* | < 0.001 |
| ≤ 15000 cells/mm3 | 126 (62.07) | 99 (78.57) | 27 (21.43) | |
| > 15000 cells/m3 | 77 (37.93) | 41 (53.25) | 36 (46.75) | |
| Neutrophils | 79.20 ± 13.20* | 76.16 ± 14.12* | 85.97 ± 7.28* | < 0.001 |
| Absolute value of neutrophils | 10.73 ± 5.28* | 9.42 ± 5.11* | 13.64 ± 4.46* | < 0.001 |
| Lymphocytes | 14.67 ± 11.80* | 17.49 ± 12.65* | 8.40 ± 6.09* | < 0.001 |
| Absolute value of lymphocytes | 1.49 ± 0.83* | 1.62 ± 0.85* | 1.20 ± 0.73* | 0.001 |
| Neutrophil-to-lymphocyte ratio | 10.81 ± 10.32* | 8.40 ± 7.93* | 16.16 ± 12.78* | < 0.001 |
| ≤ 10.4 | 124 (61.08) | 99 (79.84) | 25 (20.16) | |
| > 10.4 | 79 (38.92) | 41 (51.9) | 38 (48.1) | |
| Platelet-to-lymphocyte ratio | 269.45 ± 183.98* | 233.96 ± 139.00* | 348.32 ± 240.43* | < 0.001 |
| ≤ 284 | 134 (66.01) | 103 (76.87) | 31 (23.13) | |
| >284 | 69 (33.99) | 37 (53.62) | 32 (46.38) | |
Also, a statistically significant difference was found between the variables sex, age, fever, nausea, leukocytes, neutrophils, lymphocytes, NLR, and PLR, with perforated appendicitis (Table 1).
Predictive model
In the bivariate analysis, we found a significant association between NLR (> 10.4; OR: 3.67; 95%CI 1.97 - 6.84) and PLR values (> 284; OR: 2.87; 95%CI 1.54 - 5. 34) with perforated appendicitis, which was maintained when performing the adjusted analysis, where values > 10.4 and > 284 of NLR (OR: 2.53; 95%CI 1.27 - 5.05) and PLR (OR: 2.11; 95%CI 1.09 - 4.08), respectively, were associated with an increased risk of perforated appendicitis (Table 2).
Table 2 Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictors of acute perforated appendicitis
| Bivariate analysis | Multiple regression* | AUC | Cut-off point** | Sensitivity | Specificity | LR+ | LR- | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |||||||
| Model for NLR | ||||||||||
| NLR | ||||||||||
| ≤ 10.4 | Reference | Reference | 0.74 (0.67-0.81) | 0.29 | 77.78% | 67.14% | 2.37 | 0.33 | ||
| > 10.4 | 3.67 | 1.97-6.84 | 2.53 | 1.27-5.05 | ||||||
| Age | 0.87 | 0.79-0.96 | 0.90 | 0.81-1.00 | ||||||
| Sex | ||||||||||
| Female | Reference | Reference | ||||||||
| Male | 0.43 | 0.23-0.80 | 0.45 | 0.23-0.88 | ||||||
| Leukocytes | ||||||||||
| ≤ 15000 | Reference | Reference | ||||||||
| > 15000 | 3.22 | 1.74-5.97 | 2.01 | 0.26-3.54 | ||||||
| Model for PLR | ||||||||||
| PLR | ||||||||||
| ≤ 284 | Reference | Reference | 0.74 (0.67-0.81) | 0.29 | 77.78% | 63.57% | 2.14 | 0.33 | ||
| >284 | 2.87 | 1.54-5.34 | 2.11 | 1.09-4.08 | ||||||
| Age | 0.87 | 0.79-0.96 | 0.91 | 0.82-1.02 | ||||||
| Sex | ||||||||||
| Female | Reference | Reference | ||||||||
| Male | 0.43 | 0.23-0.80 | 0.48 | 0.24-0.92 | ||||||
| Leukocytes | ||||||||||
| ≤ 15000 | Reference | Reference | ||||||||
| > 15000 | 3.22 | 1.74-5.97 | 2.71 | 1.42-5.18 | ||||||
*Adjusted for sex, age, and leukocytes.
**Cut-off point for probability.
The estimation of the area under the curve, sensitivity, specificity, and likelihood ratio was performed using the multiple regression model.
AUC, area under the curve; CI, confidence interval; LR, likelihood ratio; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; OR, odds ratio.
When comparing the multiple regression models with and with no NLR and PLR variables, we observed an improvement in the values of the likelihood ratios and pseudo R2 when adding the NLR (with no variables: log-likelihood = -113.25, pseudo R2 = 0.099; with variables: log-likelihood = -109.76, pseudo R2 = 0.127) and PLR (with no variables: log-likelihood = -113.25, pseudo R2 = 0.099; with variables: log-likelihood = -110.82, pseudo R2 = 0.118). Considering that NLR and PLR variables contributed significantly to the multiple regression models, we decided to select the models that included the main variables.
Both models created for NLR and PLR performed well as markers of perforated appendicitis with an AUC of 0.74 (0.67 - 0.81) for both variables.
Finally, for a cut-off point of the probability of having perforated appendicitis of 30%, we observed sensitivity of 77.78% for both NLR and PLR (likelihood ratio +2.37 and +2.14, respectively), and a specificity of 67.14% and 63.57% for NLR and PLR (likelihood ratio -0.33), respectively.
Discussion
Our study evaluated a population of patients with AA to assess two potential markers for the diagnosis of perforated appendicitis. Besides abdominal pain, we found that vomiting was present in more than half of the children with AA, consistent with a previous study conducted in Turkey, where 100% and 56% of the children indicated abdominal pain and presented vomiting, respectively18. In contrast, another study in the United States reported absent symptoms in children with pathologically established appendicitis8.
While it is true that these symptoms and their severity could be helpful for diagnosis, we must consider that many of the clinical data are referred by the patient, which makes them of little use in young children due to their limited ability to communicate their symptoms20. Furthermore, there is no typical pattern in the clinical features of AA in children, so complementary diagnostic tools are required to diagnose appendicitis and detect complications such as perforation8.
Perforated appendicitis
We found perforated appendicitis in more than one-third of the population studied. Similar figures have been reported in Turkey21 and the United States7, where approximately 30% and 24% of children, respectively, presented with perforated appendicitis. The percentage found is higher than other age groups, which may be because the diagnosis of appendicitis in children is generally difficult and may progress to perforated appendicitis6,20,22.
Perforated appendicitis occurs more commonly in young children because they are less able to understand or articulate their developing symptomatology compared with adolescents. Therefore, it impacts low diagnostic accuracy in this age group5 and is associated with a delay in inpatient surgical treatment, subsequently leading to a potential risk of perforation3. Our findings corroborate this fact, as the patients with perforation were young.
A higher frequency of perforation was observed in males (39.09% vs. 21.51%), similar to a study in Germany, in which males presented with perforated appendicitis more frequently (66%)23. This higher frequency could be mainly due to differences in the immune response and differences in the characteristics of the intestinal connective tissue between males and females24,25. In this regard, it has been observed that women have higher levels of immune activation and higher gene expression associated with inflammation in intestinal mucosa samples, which, in theory, could translate into a lower incidence of perforated appendicitis cases.
Consistent with a study in China13, we also found that leukocytes (> 15000 cells/mm3), neutrophils, and lymphopenia were significantly higher in those patients with perforated appendicitis. As both leukocytes and neutrophils are part of the acute inflammatory response, their increase would be involved in the appendix inflammation process and, consequently, its perforation9. Lymphopenia is a marker of stress26 and infectious pathologies27, and its reduction is associated with the progression of appendicitis infection, especially after 6 hours28.
Markers for perforated appendicitis
NLR is a commonly available biomarker that conveys information about inflammatory conditions10 because neutrophils signal and are part of the immune response, which helps the body initiate and maintain a sustained response9. Therefore, it would be expected that the higher the NLR value, the more excessive and uncontrolled the immune response will be due to tissue destruction mediated by the inflammatory process, leading to perforation29. In this case, predictive models have reported that NLR values > 10.4 would more precisely indicate the development of perforated appendicitis18.
We evaluated the NLR considering a cut-off point of 10.4 and found a statistically significant association between NLR and perforated appendicitis, with a sensitivity of approximately 78% and a specificity of 67% for a probability of perforated appendicitis of 29%.
Although the use of NLR as a diagnostic marker in perforated appendicitis has been previously studied, primarily adult and elderly populations have been included11-13. Higher sensitivities were found in a South Korean (78%), and a Turkish (81%) study, whereas a lower sensitivity was reported in another study conducted in Turkey (64%). Additionally, these three studies reported lower specificity (66%, 53%, and 64%, respectively). These results may be because NLR measurement can potentially be impaired in adults and elderly individuals due to increased NLR when one of the following pathologies is present: high blood pressure, diabetes mellitus, metabolic syndrome30, left ventricular dysfunction, acute coronary syndrome, valvular heart disease, abnormal thyroid function, renal or hepatic dysfunction, malignancy31,32, local or systemic infection, previous history of infection (< 3 months), inflammatory disease, any medications related to the inflammatory condition and obesity17,33. In contrast, these conditions are not commonly found in pediatric patients. For this case, we found only one study that evaluated the NLR as a predictor of complicated AA in the pediatric population, which reported similar sensitivity (61%) and specificity (73%) values to those in our population18. Therefore, these findings could suggest that the use of this marker would be reproducible in different populations.
We also found a good performance of the final model, including NLR (AUC = 0.74) with relatively higher values than those reported in studies from Turkey12 and Korea11, which may be a consequence of the differences in the included population17. However, the values were similar to those reported in a pediatric population in Turkey18, with an NLR performance of only 0.71. If adjusted for other known predictors, this value could have been higher or even equal to that observed in our population.
Furthermore, we found a higher probability of perforated appendicitis for PLR values > 284, with a sensitivity of 77% and a specificity of 64% in the final model. These percentages are far from those reported in studies conducted in Turkey, both in adult13 and pediatric populations18. In general, PLR can be affected by the lymphocyte count, which is influenced by physical and psychological stress, smoking, pregnancy, and others16, or even by the platelet count, due to the sampling time, processing, and equipment used for blood analysis34. Therefore, the performance of this marker may vary under these circumstances and could present changes in sensitivity and specificity. Regardless, these values demonstrated that PLR could be a good marker for perforated appendicitis.
The hypothesis of the usefulness of this marker originates from the fact that platelets accumulate at sites of vascular injury or inflammation to maintain the leukocytes recruitment necessary for immunopathological responses. Therefore, in the presence of a more significant inflammatory response, platelets increase35 and, consequently, the PLR ratio. In this case, we found a good performance of the obtained model, including PLR (AUC = 0.74).
The adequate performance of both NLR and PLR as markers in the development of perforated appendicitis has been demonstrated. However, future studies are required to validate the proposed models, especially in longitudinal designs, for the sole purpose of verifying the performance of these markers.
Strength and limitations
The present work is one of the few studies exploring markers of appendix perforation in a pediatric population from blood tests, which are commonly used and available in emergency departments. Furthermore, NLR and PLR analysis are affordable and easy to calculate in the clinical setting, making it an effective clinical assessment tool, a valuable complement, and an aid to risk stratification.
As these markers could be used to indicate appendix perforation, which would allow determining antibiotic coverage and timely use of laparoscopic surgery, further research based on these markers should be continued.
However, some limitations should be considered. First, patients < 5 years of age were not included, so our results cannot be extrapolated to the entire pediatric population. In addition, as this was an analysis of a secondary database, some critical variables could not be included in the final model, such as the time elapsed since symptom onset.
In future research on this topic, we recommend a prospective study with a larger sample, considering some methodological, physiological, and pathological confounding factors, which could make the significance of NLR and PLR analysis in pediatric perforated appendicitis more powerful.
The present study evidenced an adequate performance of NLR and PLR as markers of perforated appendicitis. NLR values >10.4 and PLR > 284 were significantly associated with perforated appendicitis in pediatric patients. Future studies should validate the proposed models, including variables not contemplated in this study and longitudinal designs.











 text new page (beta)
text new page (beta)


