Introduction
Bartonella henselae, a Gram-negative bacterium, is the causative agent of cat scratch disease (CSD), a worldwide zoonosis1-4. The typical reservoir is the cat (particularly young kittens)1,5. B. henselae infections are thought to occur when humans are bitten or scratched by an infected cat1,2,4,6,7. It may also be transmitted by cat fleas or ticks4,6-8 or by an infected cat licking the non-intact skin of a person1,4,6,7. Cat fleas (Ctenocephalides felis) are responsible for horizontal transmission of the disease from cat to cat1,8.
Although several genotypes of B. henselae have been identified so far, two main genotypes have been designated Houston-1 (Type 1) and Marseille (Type 2). Type 1 is a commonly isolated genotype in humans from Western Europe and Australia, and it is considered more virulent1,9.
CSD affects most commonly people < 21 years of age9. Children and teenagers represent about 80% of patients diagnosed with CSD6. In the United States, the annual incidence of CSD is 4.7 per 100,000 individuals < 65 years of age and is higher among children between 5-9 years of age10. Other studies report between 54% and 87% of cases of CSD in people < 18 years of age1,11.
B. henselae infection presents as local lymphadenopathy in 85-90% of patients8, often accompanied by fever and mild constitutional symptoms1,2,6. CSD is frequently underdiagnosed due to its self-limiting course6. Extranodal manifestations with systemic or severe disease are reported in 5-20% of patients4,5. Osteomyelitis is an unusual manifestation of Bartonella observed in 0.1-0.3% of CSD patients but has been recognized to occur in immune-compromised and immune-competent patients6,12. Hepatosplenic lesions appear in 0.3-0.7% of CSD patients13,14.
Because of the difficulty to isolate B. henselae in cultures1,3,8,15, the detection of DNA by polymerase chain reaction (PCR) and serologic testing is widely used for laboratory CSD diagnosis3.
Several studies show that different antibiotics have been used to treat clinical manifestations produced by B. henselae1,5,6,8,11,12,16,17.
Clinical case
We describe the case of disseminated CSD in a previously healthy 5-year-old male. The patient came to the emergency department with a fever lasting for 5 days and exudative tonsilitis. We performed throat swab culture and then started oral amoxicillin treatment.
Three days later, the fever persisted. As the throat swab culture was negative, laboratory studies were indicated. The following findings were reported: white blood cell (WBC) count of 14.600x103/mm3 (neutrophil 8.050x103/mm3, lymphocytes 5.010x103/mm3, monocytes 1.140x103/mm3) and C-reactive protein (CRP) elevation (48.91 mg/L). Also, we performed serologic testing for adenovirus, cytomegalovirus, and Epstein Barr virus (EBV). We obtained a positive result for EBV (IgM, IgG, EBNA IgG, and heterophile antibodies) in the serologic testing. Treatment with amoxicillin was discontinued.
Two days later (10 days after the fever started), the patient presented abdominal pain. The patient had referred pain in the right hypochondrium upon palpation with no signs of abdominal resistance. The rest of the physical examination was completely normal (no hepatosplenomegaly and no lymph nodes were detected). Another blood test was done with similar results: WBC 12.960x103/mm3 (neutrophil 8.280x103/mm3, lymphocytes 3.710x103/mm3, monocytes 650x103/mm3) and CRP 64.87 mg/L. An abdominal ultrasound was requested because of the localized pain. The results showed hepatic and splenic hypoechoic lesions without hepatosplenomegaly (Figures 1 and 2). The size of the liver and the spleen were 10 cm and 8 cm, respectively, both normal for the patient's age.
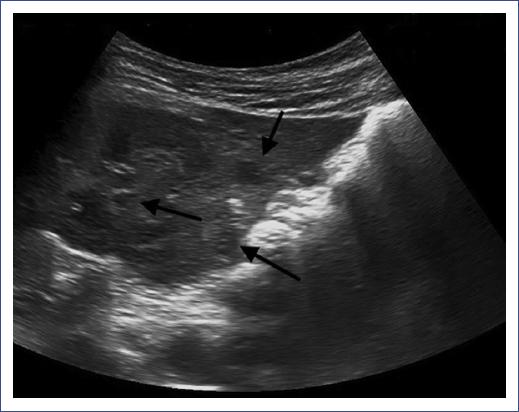
Figure 1 Hepatic ultrasound with hypoechoic lesions. Abdominal ultrasound of a 5-year-old male showing hepatic hypoechoic, not anechoic, lesions. No sign of blood flow after assessment with Doppler ultrasound is noticed. The liver has a normal size, and its outline is homogeneous.
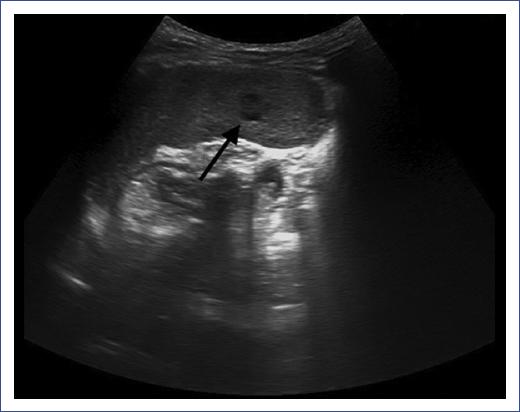
Figure 2 Spleen ultrasound with hypoechoic lesions. Abdominal ultrasound of a 5-year-old male with splenic hypoechoic, not anechoic, lesions. No sign of blood flow after assessment with Doppler ultrasound is noticed. The lesions have a maximum size of 4 mm. The spleen has normal size.
We suspected a zoonosis based on the presence of these lesions, so the family was interrogated about animals at home. The patient revealed that he had been in contact with some kittens two months before. A possible inoculation site was not found. Due to the history and lesions detected in the abdominal ultrasound, the child was admitted to our inpatient unit with a suspected diagnosis of disseminated CSD. Intravenous rifampicin and oral azithromycin were chosen while waiting for results. As the fever persisted despite the treatment, cefotaxime was added to cover other bacterial spectrums. Serologic testing was performed, looking for any zoonosis. The technique employed was indirect immunofluorescence (Focus diagnostics®). We obtained positive IgG and negative IgM of B. henselae (other zoonoses tested were negative: Rickettsia, Coxiella, Toxoplasma, Borrelia, Brucella, Francisella tularensis, and Leishmania). Blood and urine culture and stool samples were negative. An ophthalmologist and a cardiologist evaluated the patient, and no problems were detected.
Three days after admission, the patient complained of low-back pain, presenting a good response to analgesic treatment and no neurological damage. The radiography was normal. However, the orthopedist's evaluation indicated bone involvement. Therefore, the patient was transferred to a referral hospital for an MRI scan, which showed spondylitis of the D6 vertebra (Figure 3).
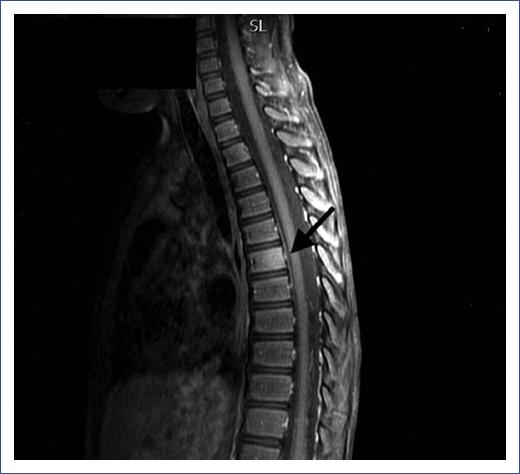
Figure 3 Magnetic resonance imaging showing spondylitis of the D6 vertebra. Magnetic resonance imaging of a 5-year-old male showing hypointensity in D6 the vertebra. After intravenous contrast administration, the vertebral body shows enhancement. These findings are suggestive of spondylitis.
The serologic testing was repeated, showing positive IgG 1:256 for B. henselae and negative IgM; also, blood PCR of Bartonella was negative. Five days later, the study was repeated with the same results. Furthermore, blood culture, immunoglobulin test, complement test, QuantiFERON, and immunodeficiencies screening were performed, and all showed normal results. A prolonged prothrombin time in the coagulation test (compared to the previous normal study) was detected. This result was interpreted as a process secondary to the acute disease. The patient was treated with vitamin K for one week after the hospitalization.
The antimicrobial therapy was changed to clindamycin and cefuroxime because of the spondylitis and the uncertainty of CSD diagnosis. Five days later, solitary adenopathy of 2 cm of diameter, painful to palpation, appeared in the upper right leg. A fine-needle aspiration (FNA) biopsy was performed, and B. henselae was identified within this lymph node (PCR positive) by immunohistochemical study.
At that point, the diagnosis of disseminated CSD with spondylitis and hepatosplenic disease was confirmed, so the treatment was changed again to intravenous rifampicin (20 mg/kg/day) and azithromycin (10 mg/kg/day). As a favorable clinical outcome was observed, the patient was discharged. The fever decreased two days after the treatment change, and the low-back pain disappeared five days later. Thus, the patient presented 2 days with fever, while it persisted 11 days after the initial onset of rifampicin and azithromycin and 9 days after cefotaxime. The patient was admitted for 17 days.
The patient follow-up was done at the first hospital. The antibiotic treatment was discontinued two weeks later for azithromycin and four weeks for rifampicin. A low-grade fever continued for three weeks. An abdominal ultrasound was done again two months after the beginning of the illness, finding that hepatic lesions disappeared, and the spleen reduced its size. Nine months later, hepatosplenic lesions and coagulation disorder disappeared.
The patient continued with fatigue for two months. After that, and until the present moment, he has been asymptomatic.
Discussion
Cat scratch disease begins with an erythematous papule at the site of inoculation. The papule appears 3 to 10 days after inoculation and progresses through erythematous, vesicular, and papular crusted stages. The lesion persists between 1 and 3 weeks1. Subsequently, there is a period of 5 to 50 days for the beginning of regional lymphadenopathy11 that usually develops 1-3 weeks after the initial skin finding1,4. The predominant clinical feature is regional lymphadenopathy, usually ipsilateral, that resolves spontaneously within 2-4 months, but it can last for several months5,8. Systemic illness is mild in most patients and can include fever, generalized aches, malaise, anorexia, nausea, and abdominal pain. Less than 10% of patients have a fever higher than 39°C, and one-third manifest the disease without fever1. The fever can last for two to eight weeks7. In the present case, it lasted for one month. Musculoskeletal manifestations, especially myalgia, arthralgia, and arthritis, occur in more than 10% of patients8.
Although infrequent, B. henselae can affect almost every organ or system after hematogenous, lymphatic, or contiguous spread6,12. Several case studies have described a wide range of atypical manifestations11,13,16,18.
Hepatosplenic involvement typically presents with dull abdominal pain, as in this case, and 53% present with hepatomegaly and splenomegaly1,17. Granulomatous lesions in the spleen can be severe enough to result in spontaneous splenic rupture1.
Regarding bone involvement, osteomyelitis typically presents as tenderness or pain in the affected area1,6,19, as in the case described here. Bartonella osteomyelitis most frequently occurs in the spine1,4,6,19. Our patient's MRI showed spondylitis of the D6 vertebra. The pelvic girdle is the most common site of Bartonella osteomyelitis outside of the spine and occurs in 42% of all non-spinal cases4,6,19. Osteomyelitis associated with CSD is usually found distant from any site of lymphadenopathy. This observation suggests a hematogenous or lymphatic spread of the infection1,4,6. Bartonella osteomyelitis usually presents subacutely in the axial skeleton, unlike hematogenous osteomyelitis, which presents acutely and typically involves the appendicular skeleton6. Patients with osteomyelitis from B. henselae infection generally have an excellent prognosis1.
Due to the possibility that other atypical manifestations were associated, an ophthalmologist and a cardiologist evaluated the patient (Table 1).
Table 1 Atypical manifestations of disseminated cat-scratch disease1,8,11
| Hepatosplenic disease |
| Parinaud's oculoglandular syndrome |
| Neuroretinitis |
| Facial nerve palsy |
| Encephalopathy |
| Seizures |
| Radiculopathy |
| Aseptic meningitis |
| Guillain-Barre syndrome |
| Cerebral arteritis |
| Transverse myelitis |
| Pneumonia |
| Osteomyelitis |
| Endocarditis |
| Glomerulonephritis |
| Renal disease |
| Arthritis/arthralgia |
| Bacillary angiomatosis |
| Thrombocytopenic purpura |
| Pseudomalignancy |
The impact of transient immunodepression during acute infectious diseases on the risk for dissemination of Bartonella infection is still debated. Underlying infections or immunological disorders should be evaluated in all cases of disseminated CSD14. Tests in our patient, immunoglobulin test, complement test, QuantiFERON, and immunodeficiencies screening were done with normal results. Regarding EBV infection detected in our case (positive IgM and IgG EBV) is possible that B. henselae may have reactivated EBV infection or EBV may have promoted the dissemination of B. henselae20.
Laboratory findings of Bartonella infection are often non-specific. Infection may result in normal or mildly elevated white blood cell counts and normal, elevated, or diminished platelet counts. Liver enzymes are usually normal. Upon admission, the levels of liver enzymes were aspartate transaminase between 21-26 U/L and alanine transaminase between 11-14 U/L in this case. The erythrocyte sedimentation rate may be normal or elevated1.
A serologic test was used to confirm the diagnosis. This test has more sensitivity than cultures but lacks specificity because many asymptomatic persons have positive serology due to previous (often asymptomatic) exposure8. Initially, when CSD was suspected, B. henselae and other zoonoses were analyzed. A positive IgG and negative IgM for B. henselae were obtained. Blood PCR can be used to differentiate Bartonella; specificity is very high, but the sensitivity is lower than serology8,16, with a reported range from 43-76%1,9,17. In our case, IgG for B. henselae was positive (1:256) and IgM negative. Blood PCR was negative.
The problem with these tests is their analysis. IgG titers greater than 1:256 strongly suggest active or recent infection6,8,16, as detected in our case. However, titers between 1:64 and 1:256 represent possible infection; another test should be performed in these patients after 10 to 14 days8. Conversely, immunoglobulin G titers less than 1:64 suggest no current Bartonella infection8,9. IgG titers decrease with time, with only 25% of patients remaining seropositive after one year1. We should not forget that a positive IgM test suggests acute infection but is often elevated only briefly (less than three months1,8,9) and is commonly normal during the disease1,6,7,12. It means that negative results do not necessarily exclude disease1,8,9. Antigenic variability between the two B. henselae serotypes may also explain the inadequate antibody response. Commercial kits use only B. henselae strain Houston-1 grown in cell culture as the antigen. Antibodies directed against the B. henselae Marseille strain may remain undetected9.
PCR testing of blood specimens can detect B. henselae DNA in patients with seronegative results. The combined use of serologic testing and PCR on blood specimens is helpful for the noninvasive screening of CSD3.
Finally, in our case, B. henselae was identified from a lymph node (PCR positive). The confirmatory test is PCR or direct visualization of B. henselae on biopsied material from the site of involvement16. Isolation of bacteria from lymph node culture also offers poor yield because lymphadenopathy is thought to be the consequence of an aggravated immune response rather than the bacterial direct invasion and proliferation1,9.
Micro-abscesses in the liver or spleen can be detected in more than 50% of patients with hepatosplenic CSD through abdominal imaging studies17. Multiple hepatosplenic lesions are usually small, round, and hypodense on computerized tomography and result from granulomas, abscesses, or lymphoplasmacytic inflammation13.
Osteomyelitis lesions are sometimes subtle on the plain X-rays and may require an MRI or radionuclide bone scan to be found1,6.
In this case, hepatosplenic lesion diagnosis was performed by ultrasound and the spondylitis of the D6 vertebra by MRI.
Treatment of CSD depends on the disease presentation. Most patients usually do not require antibiotics because CSD typically resolves within weeks to months regardless of antimicrobial therapy due to its self-limiting nature6,12,16. In atypical or complicated CSD, including osteomyelitis and hepatosplenic disease, the optimum treatment and duration have not been established5,16,17. Current knowledge on treating atypical or complicated CSD is derived from the observation of case studies rather than randomized trials1,11. In the literature, some cases of disseminated CDS resolved without treatment21, and others progressed despite antimicrobial therapy4.
In vitro, Bartonella species are susceptible to macrolides, aminoglycosides, B-lactams, expanded-spectrum cephalosporins, trimethoprim-sulfamethoxazole (cotrimoxazole), rifampicin, and ciprofloxacin1. All antibiotics tested in vitro had only bacteriostatic activity, except aminoglycosides, which demonstrated bactericidal activity1,16.
Azithromycin is used as a first-line agent for lymphadenopathy4,6,8,12. Single reports on the effectiveness of different antibiotic treatments in CSD suggest the usefulness of azithromycin (10 mg/kg/day), clarithromycin (10-15 mg/kg/day), gentamicin (5 mg/kg/day), ciprofloxacin (20-30 mg/kg/day), rifampicin (20 mg/kg/day) and cotrimoxazole (40 mg/kg/day of sulfamethoxazole)11,13,16,19.
For patients with osteomyelitis, the recommended treatment is high doses of ciprofloxacin, doxycycline, or azithromycin for extended periods (6-12 weeks, minimum 4 weeks), 10 to 14 days of intravenous treatment followed by oral treatment. The use of two antimicrobial agents together is also recommended11. In systemic CSD, a combination of gentamicin and an oral agent such as cotrimoxazole, rifampicin, ciprofloxacin, or azithromycin has demonstrated a favorable response1,17.
In our case, when CSD was suspected, azithromycin and rifampicin were started while waiting for the results of laboratory tests. Later, when the fever persisted and the child referred back pain, we decided to associate a third-generation cephalosporin for targeting other bacteria. Because of the spondylitis and the CSD diagnosis uncertainty, the antimicrobial therapy was changed to clindamycin and cefuroxime. When the diagnosis was confirmed, azithromycin and rifampicin were restarted for two and four weeks, respectively. Thus, including the treatment received during admission, the duration of azithromycin was 4 weeks and rifampicin 6 weeks (with 15 days of intravenous treatment for both drugs). Azithromycin was chosen to treat osteomyelitis, and it was discontinued 4 weeks later because the low-back pain disappeared. Rifampicin was chosen to treat systemic CSD and was kept for 6 weeks because the low-grade fever and the fatigue continued.
It has also been described that corticosteroid therapy may be helpful in patients with hepatosplenic CSD and prolonged fever22.
The fever can last for two to eight weeks7. Our patient presented low-grade fever for three weeks and weakness for two months (possibly related to the EBV infection). In general, symptoms and hepatosplenic lesions remit within 6 months1,13. However, there have been rare reports of residual calcification1. In this regard, the abdominal ultrasound was normal nine months after the disease in our patient.
The literature search using PubMed MeSH headings "cat scratch disease" and "osteomyelitis" revealed 54 publications, which were reduced to 42 when limiting articles to the population under 18 years of age. If we further limited the search for patients with "hepatosplenic lesions," no publications were available.
Human infection most commonly manifests as regional lymphadenopathy. Although any organ can be involved after bacteremia, osteomyelitis due to B. henselae is a rare presentation2.
The diagnosis of disseminated B. henselae is based on elevated titers, radiologic findings, and epidemiologic history1,6,17.
The combined use of serologic testing and PCR on blood specimens is useful for the noninvasive screening of CSD3.
In atypical or complicated CSD, including patients with osteomyelitis and hepatosplenic disease, the optimum treatment and duration have not been established5,16,17.
The clinical case presented in this article is infrequent, as indicated by the currently available literature.











 nueva página del texto (beta)
nueva página del texto (beta)


