Introduction
The world is currently experiencing one of the most significant public health pandemics caused by the outbreak of the new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019 [COVID-19] and 2019-nCoV), with a significant impact due to its high mortality. COVID-19 infection has spread to all regions of the world, affecting nearly 208 countries. So far, 9,178,773 confirmed cases of the infection, 474,513 deaths, and 4,595,846 recoveries have been reported (June 23, 2020), making SARS-CoV-2 the third highly pathogenic coronavirus infecting humans in the 21st century1. Because of this, the World Health Organization (WHO) declared the outbreak of the new coronavirus an emergency of international concern2-4.
The SARS-CoV-2 outbreak is reported to have started in December 2019 in China (Wuhan, Hubei), where cases of severe pneumonia of unknown cause were reported. The initial cases had a common exposure: people who traded live animals at the Wuhan seafood and wildlife market3-5. According to the Chinese epidemiological surveillance system, the report of respiratory samples from infected persons corresponded to a coronavirus, which showed more than 95% similarity to bat coronavirus (BtCoV/4991) and more than 70% similarity to SARS-CoV3,4,6. Although the evidence suggests that SARS-CoV-2 originated from bats, it is believed that the intermediary animals through which it passed to humans were pangolins and snakes. A genomic analysis of a coronavirus isolated from pangolins showed 99% similarity in the strains isolated compared to SARS-CoV-2; however, the origin of the virus remains uncertain3-5,7.
Although the spread of COVID-19 is global, clinical and epidemiological patterns remain unclear, especially in the pediatric population. SARS-CoV-2 is transmitted from person to person through respiratory droplets from the mouth or nose or direct contact6. According to several studies, it is estimated that the infection has an average incubation period of 4 days (range: 2-7 days), with the possibility of asymptomatic transmission. Similarly, it is estimated that the average duration of the infection from its start until hospital admission is approximately 12.5 days, which means that, on average, each patient is capable of spreading the infection to 2.2 other people8-10. The average age of patients infected by SARS-CoV-2 is around 47 years (range: 35-58), and the male population predominates in the number of those infected (56-61.9%), with a small number of cases in children under 15 years6,8,11,12. The SARS-CoV-2 infects the human lungs alveolar epithelium cells, causing SARS. Among the main clinical features present in infected patients are fever (> 90%), cough (70%), dyspnea, myalgias, and headache; however, rhinorrhea, sore throat, nausea, vomiting, and diarrhea may also be present in a lower proportion. During hospitalization, most patients develop severe pneumonia (> 90%), even leading to septic shock in some cases6,10-12. It has also been demonstrated that having some underlying comorbidity (hypertension, cardiovascular disease, diabetes mellitus, and chronic obstructive pulmonary disease, among others) and being over 60 years old are risk factors for developing severe disease6,11. It is estimated that the average length of hospital stay is approximately 12 days11.
Until now, there has been no effective treatment to treat COVID-19. There are several drugs that have been used as possible candidates, including lopinavir/ritonavir, neuraminidase inhibitors, remdesivir, umifenovir, inhibitors of DNA (tenofovir, disoproxil, and lamivudine) and RNA synthesis (TDF, 3TC), chloroquine, and nucleoside analogs, and even traditional Chinese medicine. Although some of the drugs suggest a favorable clinical response, further clinical studies are still required to assess their potential therapeutic efficacy and safety6,13,14.
Public health and infection control measures are required to limit COVID-19 spread and reduce the harm associated with it. Therefore, based on previous experience in managing Middle East respiratory syndrome (MERS) and SARS infections, the WHO recommends using interventions to reduce and interrupt the risk of transmission of acute respiratory infections2,15. Recommended measures include avoiding close contact with people with respiratory infections, frequent hand washing, barrier measures such as masks, improving infection prevention and control practices in hospitals, and avoiding unprotected contact with farm or wild animals2,6,15-17.
The COVID-19 pandemic has posed significant challenges globally. Undoubtedly, the viruss continued transmission is mainly due to the lack of vaccines and therapeutic agents. Although countless efforts have been made to develop vaccines against SARS-CoV and MERS-CoV infections in the last decade, so far none has been approved for use in humans4,7. The process of vaccine development means that the organism acquires some immunity to the new pathogens, in this case, to SARS-CoV-2. To understand how vaccines should function, it is necessary to understand the coronavirus infection process, which begins when the surface protein (spike [S]) binds to receptors for the angiotensin-converting enzyme 2 on the surface of human cells. Once the virus invades the host cells, the latter translates the viral RNA, modifying its genetic information and becoming cells that generate new viruses (viral replication)4,7,18,19. Therefore, the process of developing vaccines entails that they act on the mechanism of infection of SARS-CoV-2. Several studies have been initiated to create a vaccine to prevent SARS-CoV-2, which would cover the vaccination of populations at risk. The situation demands strategies to stop the diseases spread. Therefore, this review aimed to identify and describe possible vaccines for the prevention of COVID-19.
Methods
A systematic search of the literature in three different electronic databases (PubMed, Trip Database, and Cochrane Library) was conducted from December 1, 2019, until June 19, 2020, to identify reports or published studies on SARS-CoV-2 (2019-nCoV) developing vaccines. In addition, a complementary search was conducted in other sources of information: web sites of international organizations, health and research institutes, Google, and the references of the identified studies. The following keywords were used for the search: COVID-19, 2019-nCoV, SARS-CoV-2, COVID-19, coronavirus infection, SARS, novel COVID-19, coronavirus Wuhan, vaccine, vaccination, vaccinate, prevention. In PubMeds search engine, methodological filters were used: review, systematic review, clinical trial, randomized controlled trial, and MeSH terms. The search was not limited by language. Studies examining the mechanisms of infection, immunopathological mechanisms, and genomics were excluded from the study.
Results
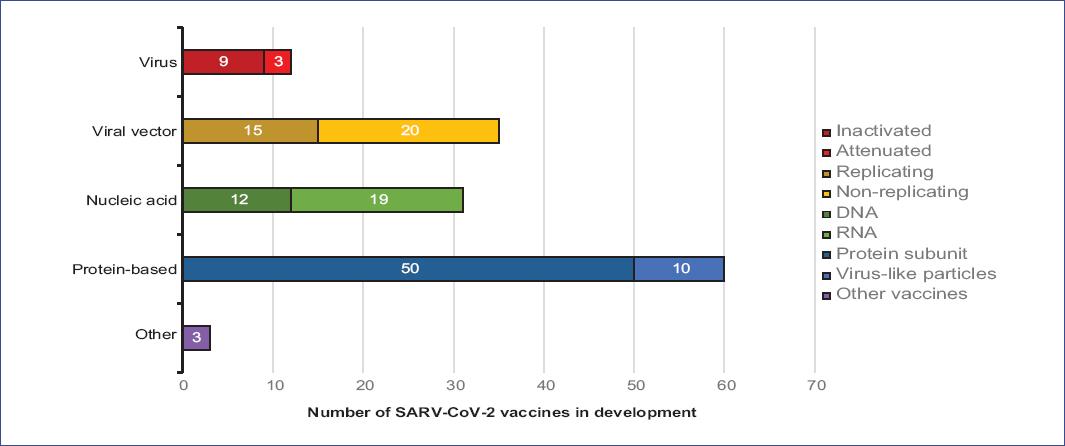
According to the information currently available (June 19, 2020), 141 probable SARS-CoV-2 vaccines are being developed worldwide, of which only 13 vaccines (9.2%) are in the process of clinical evaluation (Table 1). Research teams from various companies, research institutes, and universities test different technologies to develop these vaccines. Many of these technologies have not been used before in an approved vaccine19-22. Among the different types identified, we found virus vaccines (weakened [attenuated] and inactive), viral vectors (replicative and non-replicative viruses), nucleic acid (DNA and RNA), and protein based (protein subunit and virus-like particles [VLP]) vaccines (Figure 1). The different types of vaccines identified are described below.
Table 1 Candidate vaccines in the process of clinical evaluation22
| Organization | Vaccine | Technological platform | Type of vaccine | Coronavirus target | Current stage of clinical evaluation | U.S. Food and Drug Administration regulated pharmaceutical product | Same platform for candidates other than coronavirus |
|---|---|---|---|---|---|---|---|
| University of Oxford/AstraZeneca | AZD1222 | Non-replicating viral vector | ChAdOx1-S (ChAdOx1 nCoV-19) | SARS-CoV-2 | Phase 2b/3 2020-001228-32 Phase 1/2 2020-001072-15 |
| MERS, influenza, TB, Chikungunya, Zika, MenB, plague |
| CanSino Biological Inc./Beijing Institute of Biotechnology | Ad5-nCoV | Non-replicating viral vector | Adenovirus type-5 vector | SARS-CoV-2 | Phase 2 ChiCTR2000031781 Phase 1 ChiCTR2000030906 |
| Ebola |
| Wuhan Institute of Biological Products/Sinopharm | Vero cells | Inactivated | Inactivated | SARS-CoV-2 | Phase 1/2 ChiCTR2000031809 |
| |
| Beijing Institute of Biological Products/Sinopharm | Vero cells | Inactivated | Inactivated | SARS-CoV-2 | Phase 1/2 ChiCTR2000032459 |
| |
| Sinovac | CoronaVac | Inactivated | Inactivated + alum | SARS-CoV-2 | Phase 1/2 NCT04383574 NCT04352608 |
No | SARS |
| Institute of Medical Biology, Chinese Academy of Medical Sciences | Unknown | Inactivated | Inactivated | SARS-CoV-2 | Phase 1 NCT04412538 | No | |
| Novavax | NVX-CoV2373 | Protein subunit | Recombinant SARS CoV-2 nanoparticle glycoprotein vaccine with adjuvant matrix M | SARS-CoV-2 | Phase 1/2 NCT04368988 |
No | RSV, CCHF, HPV, VZV, EBOV |
| Moderna/National Institute of Allergy and Infectious | mRNA-1273 | RNA | LNP-encapsulated mRNA | SARS-CoV-2 | Phase 2 NCT04405076 Phase 1 NCT04283461 |
Yes | Multiple candidates |
| BioNTech/Fosun Pharma/Pfizer | 3 LNP-mRNAs | RNA | 3 LNP-mRNAs | SARS-CoV-2 | Phase 1/2 2020-001038-36 NCT04368728 | Yes | |
| Inovio Pharmaceuticals | INO-4800 | DNA | Electroporation of plasmid DNA | SARS-CoV-2 | Phase 1 NCT04336410 |
Yes | Multiple candidates |
| Gamaleya Research Institute | Unknown | Non-replicating viral vector | Adeno based | SARS-CoV-2 | Phase 1 | | |
| Imperial College London | Unknown | RNA | saRNA | SARS-CoV-2 | Phase 1 | | EBOV, LASV, MARV, Inf (H7N9), RABV |
| CureVac | CVnCoV | RNA | mRNA | SARS-CoV-2 | Phase 1 | | RABV, LASV, YFV, MERS, InfA, ZIKV, DENV, NIPV |
CCHF: Crimean-Congo hemorrhagic fever; DENV: dengue virus; EBOV: Ebola virus; HPV: human papillomavirus; LASV: Lassa virus; MARV: Marburg virus; MenB: meningitis B; MERS: Middle East respiratory syndrome; NiPV: Nipah virus; RABV: rabies virus; RSV: respiratory syncytial virus; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; TB: tuberculosis; VZV: varicella-zoster virus; YFV: yellow fever virus; ZIKV: Zika virus.
Virus vaccines
There are 12 projects on virus vaccines, from which nine are using the SARS-CoV-2 virus in its inactive form and three are based on attenuated live viruses. In one of them, the measles virus is being used. Of the total number of vaccines, four inactivated virus vaccines are in the clinical stage with Phase I-II studies. The other eight vaccines are still in the preclinical evaluation process and are developed by biotechnology companies, research institutes, and biological products companies from India, Kazakhstan, France, Germany, Japan, China, and the USA19,22.
In Beijing, Sinovac Biotech began testing a vaccine made from an inactive version of SARS-CoV-2 in humans after publishing preclinical results of its vaccine candidate (CoronaVac)22-24. According to the preclinical study, the inactive virus vaccine induced neutralizing antibodies specific for SARS-CoV-2 in mice, rats, and rhesus monkeys. The results showed that CoronaVac offered safe and complete protection against SARS-CoV-2 strains in rhesus monkeys24. In April 2020, Sinovac started a double-blind, placebo-controlled, randomized clinical trial for its COVID-19 (CoronaVac) candidate vaccine in humans, in which 144 healthy adults between 18 and 59 years old were included in the study. The study aimed to evaluate the safety, tolerance, and immunogenicity of the vaccine. Sinovac Biotech intends to generate up to 100 million doses of CoronaVac annually, for which it is building facilities for manufacturing23.
Furthermore, Sinopharm (China National Pharmaceutical Group Co., Ltd.), together with the Wuhan Institute of Biological Products, proposed a randomized, double-blind, placebo-controlled clinical trial to evaluate the safety and immunogenicity of the inactivated coronavirus vaccine (Vero cells) in a population of almost 100 healthy people over 3 years of age. Sinopharm is also conducting another trial of similar characteristics using an inactive virus vaccine (Vero cells) in collaboration with the Beijing Institute of Biological Products22,25. Moreover, the Institute of Medical Biology of the Chinese Academy of Medical Sciences also proposed a SARS-CoV-2 inactive virus vaccine, which is currently undergoing Phase I-II clinical evaluation. A randomized, double-blind, placebo-controlled clinical trial is underway, including 942 healthy individuals aged 18-59 years to evaluate the safety and immunogenicity of the vaccine, which will be sponsored by the Chinese Academy of Medical Sciences. It is worth mentioning that one of the achievements of the academy has been the generation of the vaccine against polio and hepatitis A22,26,27.
Viral vector vaccines
We found 35 different viral vector vaccines against SARS-CoV-2 in development. These vaccines consist of genetically modified viruses with the information needed to produce coronavirus proteins in the body. The modified virus is weakened to avoid causing the disease. Of these vaccines, 20 are non-replicating viral vectors (key genes have been deactivated to prevent replication), and 15 are replicating viral vector vaccines, in which viruses can still replicate inside the cells19,20,22.
The University of Oxford, in collaboration with AstraZeneca, developed the project of a non-replicating viral vector vaccine against SARS-CoV-2, called AZD1222 (ChAdOx1 nCoV-19). This vaccine is based on modified viruses, in which genetic sequences are introduced for the production of coronavirus spike protein (S protein) with the objective that the immune system recognizes this protein and acquires immunity against SARS-CoV-2 without causing infection. For this vaccine, an inactivated adenovirus obtained from chimpanzees was used as a vector. AZD1222 is in Phase I-II and clinical evaluation through a controlled clinical trial that aims to determine the vaccines efficacy, safety, and immunogenicity19,28-30. The Phase I study began in April of 2020, including 1112 healthy adult volunteers between 18 and 55 years of age from the United Kingdom. The ChAdOx1 nCoV-19 will be compared against Pfizers Nimenrix (MenACWY) vaccine, which will be used as a control. Nimenrix aims to protect against infections caused by Neisseria meningitidis types A, C, Y, and W-135.
Moreover, up to 10,260 participants are expected in the ChAdOx1 nCoV-19 Phase II project, including children from 5 to 12 years old and adults over 56 years old. In Phase III, the inclusion will be limited to persons over 18 years of age. In both phases, the interventions allocation will be random19,22,28-30.
CanSino Biologics Inc., in collaboration with the Beijing Institute of Biotechnology, is working on another non-replicating viral vector vaccine against SARS-CoV-2: the so-called Adenovirus Type 5 Vector (Ad5-nCoV)22,31. According to preclinical studies, Ad5-nCoV showed to be one of the vaccine candidates since it can induce a robust immune response in animal models. Similarly, preclinical studies showed a good safety profile; hence, it is currently in Phase II clinical evaluation31. To evaluate the safety, tolerability, and immunogenicity of Ad5-nCoV in humans, a single-center, open-label, Phase I, a dose-escalating clinical trial involving 108 healthy adults 18-60 years was conducted. It should be pointed out that this was the first clinical study of COVID-19 vaccines carried out in humans, which began on March 16, 202025,27,31,32. According to the trial results, the report concludes that the Ad5-nCoV vaccine is tolerated and immunogenic 28 days after vaccination. The humoral responses against SARS-CoV-2 reached their peak on day 28 after vaccination in healthy adults31,32. Therefore, a randomized, double-blind, placebo-controlled, Phase II clinical trial will be developed to evaluate the new recombinant vaccines safety and immunogenicity (Ad5-nCoV) in a population of 500 healthy adults over 18 years of age25,31. Meanwhile, the Gamaleya Research Institute in Russia is developing a non-replicating adenoviral vector-based vaccine, which is currently undergoing Phase I clinical evaluation. Thus, at least seven research groups are conducting a clinical trial in which 76 people will participate22,33.
The remaining non-replicating viral vector vaccines against SARS-CoV-2 (17 vaccines) and the 15 proposals of replicating viral vector vaccines are still in the preclinical phase22.
Nucleic acid vaccines
At present, 31 genetic SARS-CoV-2 vaccines based on nucleic acids have been reported and are underdevelopment. This type of vaccine aims to use genetic instructions in a DNA or RNA form to induce an immune response. Their mechanism of action is to insert nucleic acids into human cells, which will produce copies of the virus protein. Most of these vaccines encode the virus spike protein, which is the protein that coats the virus and allows it to adhere to the external surfaces of human cells19-21. Of the total number of vaccines, 19 are RNA and 12 are DNA; so far, only five of the vaccines are under clinical evaluation19,22.
The U.S. National Institute of Allergy and Infectious Diseases and a biotechnology company, Moderna, are developing a messenger RNA vaccine (mRNA-1273) originally designed to prevent MERS but adapted for SARS-CoV-234. This vaccine is encapsulated in lipidic nanoparticles that encode the stabilized SARS-CoV-2 S protein (the coronaviruses main surface antigen). It is currently in Phase I-II of the clinical evaluation process20,22,35. On March 16, 2020, a non-randomized, open-label, Phase I clinical trial initiated with 155 healthy volunteers over 18 years to evaluate whether the mRNA-1273 vaccine is safe and induces an immune response27,34,35. Recently, Moderna announced preliminary results on the study, in which positive results were suggested for mRNA-1273, indicating that the vaccine is safe and well tolerated. Besides, mRNA-1273 produced high levels of neutralizing antibodies34. Furthermore, on May 12, Moderna Therapeutics received the Fast Track designation from the U.S. Food and Drug Administration (FDA) for its COVID-19 candidate vaccine. As a result, a Phase II study is being planned, which will evaluate the safety, reactogenicity, and immunogenicity of the mRNA-1273 vaccine compared with placebo; the study will include 600 healthy people > 18 years old. Similarly, a Phase III clinical trial is expected to start in July 202034,35.
The companies BioNTech, Fosun Pharma, and Pfizer also joined forces to develop another RNA vaccine: the so-called 3 LNP-mRNAs (biological: BNT162a1), which is in the process of clinical evaluation22. A Phase I-II randomized controlled placebo-controlled clinical trial was proposed to evaluate the safety, tolerability, immunogenicity, and potential efficacy of the 3 LNP-mRNAs vaccine against CoV-2 RNA (COVID-19). The study began on April 29, 2020, and aimed to include 7600 healthy participants aged 18-8527,29. Imperial College London and the biopharmaceutical company CureVac also proposed two COVID-19 vaccines based on RNA (saRNA, mRNA), which are in the Phase I clinical evaluation process22,36. CureVac obtained approval from the German Health Authority Paul-Ehrlich-Institute and the Belgian Federal Agency for Medicines and Health Products to begin its clinical trial. The study will be conducted in Germany and Belgium and will include 168 healthy subjects from 18 to 60 years old to evaluate the optimal dose, safety, and immune profile of the CVnCoV vaccine (mRNA) in humans. The Federal Ministry for Economic Affairs and Energy announced that it would invest 300 million euros in CureVac36.
Moreover, the candidate vaccine from Imperial College London has undergone rigorous safety testing in its preclinical phase, demonstrating an effective immune response, for which a Phase I clinical trial will be initiated, including 300 healthy persons from 18 to 75 years of age to test the optimal vaccine dose. Imperial College London has proposed that if the vaccine proves to be safe and produces a favorable immune response in humans, a Phase III clinical trial with 6000 healthy volunteers will be undertaken to test its effectiveness. It is expected that a viable vaccine may become available in the spring of 202137.
Separately, Inovio Pharmaceuticals intends to develop a vaccine against SARS-CoV-2, named INO-4800, based on DNA (DNA plasmid vaccine with electroporation). Inovio has vast experience developing DNA-based technologies, as it was the first company to advance toward a vaccine against MERS-CoV. For this reason, on January 10, the company started the preclinical tests to evaluate the immune response provided by the INO-4800 vaccine in animal models22,27,38. After the immunization of mice and guinea pigs with INO-4800, it was possible to measure functional antibodies that neutralize SARS-CoV-2 infection and T-cell response to the specific antigen. Based on the results obtained, INO-4800 promises to be a possible COVID-19 vaccine candidate38,39. The development process toward the clinical trials of INO-4800 has been accelerated. On April 6, 2020, the beginning of the first open, non-randomized Phase I clinical trial to evaluate the vaccines safety, tolerability, and immunogenicity was announced. The study was authorized by the FDA and aimed to include 120 participants. Similarly, the next Phase II-III studies have been planned. Inovio has the support of the Bill & Melinda Gates Foundation and the U.S. Department of Defense22,27,38.
Protein-based vaccines
At least 50 proposed SARS-CoV-2 vaccine formulations are composed of viral protein subunits; most focus on the SARS-CoV-2 spike protein, or an essential part called the receptor blinding domain22. Similar vaccines have been developed against SARS; however, these have not yet been tested in humans7,19. So far, only one of the viral protein formulations is under clinical evaluation: the NVX-CoV2373 vaccine (biological: SARS-CoV-2 rS), developed by the biotechnology company Novavax. NVX-CoV2373 is a recombinant full-length SARS-CoV-2 glycoprotein nanoparticle vaccine with Matrix-M adjuvant, underevaluation of its immunogenicity and safety through a Phase I-II randomized controlled clinical trial. The study aims to evaluate the vaccine against placebo, including healthy participants aged 18-59 years27,40. According to preclinical tests, NVX-CoV2373 demonstrated positive results in baboons, which showed an immune response similar to convalescent plasma in humans, demonstrating strong immunogenicity and high protection40.
Virus-like particles vaccines
Within the developed technologies, there are also proposals in which fragments of proteins or protein layers that mimic the coronaviruss outer layer are used, which generally lack genetic material. To date, 10 proposals for vaccines based on virus-like particles (VLP) have been developed, including a proposal made from plant derivatives. All of them are still in the preclinical evaluation process19,22. In addition, other vaccines are being developed, of which the type of technology used is unknown since there are no reports. However, it is known that they are in the preclinical evaluation process. Some biotechnology companies are testing existing vaccines, such as those for polio and tuberculosis, as it is thought that these could induce a general immune response rather than specific adaptive immunity19-21.
Thirteen controlled clinical trials are currently underway, of which seven report the use of placebo. Six are randomized studies, two are non-randomized, four are double-blind, and two are open-label studies22,25,27,29.
Discussion
With the emergence of the new SARS-CoV-2 coronavirus, the world faces one of the most impactful health emergencies in history. One of the principal needs is to control the current pandemic, so developing an effective and safe vaccine is one of the challenges to be solved.
A few decades ago, vaccines production against viral diseases included immunization with live-attenuated or inactive viruses. Today, advances in biotechnology and genomics have changed the perspective in developing new vaccines since they are based mainly on antigens and immunogens. At present, different types of technologies are being used for the generation of vaccines against coronavirus. Among the technologies used are those based on proteins with the application of subunits, genetic vaccines based on nucleic acids, and inactivated, vectorized, and attenuated live virus vaccines.
The development of a coronavirus vaccine began after the SARS-CoV outbreaks in 2003 and MERS-CoV in 2013, with the emergence of several proposed technologies. However, no vaccine for humans has been approved despite countless efforts to generate an effective and safe vaccine for both SARS and MERS4,7,21.
To date, 141 SARS-CoV-2 candidate vaccine proposals have been submitted. Various international organizations, private companies, educational institutions, and research institutes make great efforts to generate a clinically useful vaccine. However, only 13 of these vaccines are in the process of clinical evaluation. For all these vaccines, clinical trials are being conducted in healthy individuals to evaluate their immunogenicity, tolerability, safety, and potential efficacy. Five vaccines are currently in Phase I studies, and seven vaccines are in Phase I-II studies. Only the vaccine elaborated by the University of Oxford and AstraZeneca (AZD1222) is in clinical evaluation Phase II-III. The clinical trials for these phases will be carried out in England and Brazil, where more than 10,000 people will be immunized28-30. According to reports, AstraZeneca plans to deliver emergency vaccines on September, 2020, with the possibility of manufacturing up to 400 million doses. The Oxford researchers had previously used the same virus used in the AZD1222 vaccine to develop a vaccine against MERS-CoV, which served for accelerating the new SARS-CoV-2 vaccine since the previous studies had demonstrated its safety28-30.
Another promising proposal is Ad5-nCoV, a non-replicating viral vector vaccine against SARS-CoV-2 developed by CanSino Biologics and the Beijing Institute of Biotechnology. According to the available information, the first studies carried out in Wuhan (China) showed an immune response after 28 days of the vaccine administration, with the production of two neutralizing antibodies in humans31,32. However, the levels of neutralizing antibodies that the body requires to protect itself from SARS-CoV-2 infection are unknown. Therefore, these results should be interpreted with caution since the vaccines ability to trigger an immune response does not indicate that it protects against SARS-CoV-2. Although CanSino was the first company to conduct a clinical study of a COVID-19 vaccine, it is currently still in clinical evaluation (Phase II). Ongoing clinical trials are being conducted in China and Canada, with final results expected in 6 months. However, there are still reserves with Ad5-nCoV since, when using an adenovirus that frequently produces the common cold, there is a possibility that it will produce strong antibody responses when inoculated in high doses, which can be toxic. Therefore, CanSino has considered the possibility of using only low and medium doses31,32.
Another technology that has allowed the rapid design and development of a vaccine is the one used in the vaccine proposed by the U.S. company Moderna, based on messenger RNA (mRNA-1273). The results of studies carried out suggest that mRNA-1273 stimulates the production of neutralizing antibodies. As it is a safe vaccine, it has obtained the Fast Track designation by the FDA. Today, mRNA-1273 is in Phase II clinical evaluation. However, it is expected that in July 2020, Phase III trials will start, in which up to 30,000 people are expected to be included. In case that the results of Phase III are favorable, Moderna anticipates the beginning of the mass production of the vaccine for September, 2020, with the possibility of having 1 billion doses ready by mid-202134,35.
There are some research reports about COVID-19. Many of them cover both prevention and treatment21,41,42; others focus on immunopathological and genomic mechanisms43-45. However, no review analyzing the different vaccines in development against COVID-19 has been conducted from a general perspective. Our study coincides with some of the reports generated in the different vaccine tracking platforms19 and the Coalition for Epidemic Preparedness Innovations (CEPI) analysis20. CEPI is currently working with different world health authorities and vaccine developers to support the elaboration of vaccines against COVID-1946.
The New York Times recently generated a platform where it presents updated and summarized information on the different vaccines against COVID-1947. Similarly, the Milken Institute of the United Kingdom48 informs the last updates on the different treatments and vaccines against COVID-19. However, none of these repositories provide all the current information on each vaccine since they only generate one record. The WHO has also updated this information and listed each of the proposals and their clinical evaluation status22. With this review, we intend to provide updated information about the different vaccines proposals against COVID-19, for which we have considered the existing scientific evidence.
Significant efforts have been made in the research and development of vaccines against COVID-19 worldwide. Given the need regarding timing, it has been established that vaccines could be available under emergency use by early 2021. For this reason, new guidelines will be necessary for its development, considering all its phases and the process of adaptation, regulation, and manufacture together.
Despite efforts to generate a SARS-CoV-2 vaccine and potential proposals with promising results, additional research and biotechnological development are still needed. It is essential to address the new formulations various challenges, thus ensuring an effective vaccine. In addition to the technical difficulties related to the vaccines production, difficulties associated with their development should also be resolved.
Different international health organizations must get involved in the vaccines approval and implementation processes since more than 70% of the groups leading the vaccine research efforts are private companies.
Action plans need to be established in different settings and countries to access potential vaccines, thus ensuring the current COVID-19 pandemic management. Joint and global efforts are needed to counteract the collateral damage of this deadly disease.











 nueva página del texto (beta)
nueva página del texto (beta)