Introduction
Pediatric acute myeloid leukemia (AML) is a heterogeneous disease. Despite intensive chemotherapy, the outcome of AML reaches a plateau, with 5-year event-free survival (EFS) rates ~60% and 5-year overall survival (OS) rates reaching 70%1-3.
Apart from the early clinical response, the best-recognized factors for survival are cytogenetic and molecular aberrations4.
The identification of prognostic subgroups is important to establish a treatment stratification system. Therefore, the investigation of specific genetic subgroups of very low prevalence requires international collaboration to allow individual study groups and evaluate prognostic significance.
A pediatric AML group of concern is t(16;21), which is considered high risk in agreement with the existing literature. This condition is mainly found in case reports or small series of adult patients5-9.
The t(16;21) subgroup was primarily described as a therapy-related condition that showed poor prognosis in adults; however, in pediatric AML, this translocation was quite rare and tended to be de novo AML9.
Two different t(16;21) translocations resulting in different fusion transcripts have been reported. These include t(16;21)(p11;q22), resulting in the FUS-ERG fusion, and t(16;21)(q24;q22), resulting in the RUNX1-CBFA2T3 (core-binding factor) fusion10.
Large cooperative pediatric groups have carried out a series of case studies trying to gather these patients and analyze their clinics. The group with the most pediatric cases is the international Berlin-Frankfurt-Münster (I-BFM) Study Group11. They identified 55 pediatric patients with t(16;21) in a retrospective analysis over 21 years, including 32 patients with FUS-ERG-rearranged AML (two of them with secondary AML) and 23 patients with RUNX1-CBFA2T3-rearranged AML. The 4-year EFS rate of patients with FUS-ERG AML was 7% (standard error [SE] 5%), which was significantly lower than that of the reference cohort (51%, SE 1%, p < 0.001). The 4-year EFS rate of RUNX1-CBFA2T3 AML was 77% (SE 8%, p = 0.06), which was significantly higher than that of the reference cohort11.
From this series, isolated cases with heterogeneous clinical responses have been reported9,12,13.
We report one case of a patient with AML M6 with t(16;21) FUS-ERG-rearranged AML. To the best of our knowledge, this is the first association reported between this translocation and this morphological subtype of the French-American-British (FAB) classification.
Clinical case
In 2019, a previously healthy 13-year-old female was admitted because of asthenia and fatigue of 1-month evolution associated with unquantified intermittent fever. The patient showed pruritus, erythema, and disseminated dermatosis, which consisted of millimetric erythematous papules, some with a hypopigmented area, in the anterior thorax, abdomen, and lower extremities. We detected skin papules with short hair of follicular appearance of subacute evolution. Hepatomegaly was present as well. Blood count on admission revealed the following results: hemoglobin level, 9.7 g/dL; hematocrit, 28.6%; white blood cell count, 20,900/mL; neutrophil count, 9.6%; and platelet count, 69,000/mL. Lactate dehydrogenase, transaminase, and uric acid levels were within the normal range.
On January 11, 2019, bone marrow (BM) aspiration was performed, of which 82.5% were blast cells and > 50% were erythroblasts; dyserythropoiesis was observed. According to FAB classification, a diagnosis of AML type 6 was made based on the BM examination. Blast cells were negative to myeloperoxidase staining and positive to periodic acid–Schiff (PAS) staining (Fig. 1). The following positive expression results were observed by immunohistochemistry: immunophenotype CD10 (2.0%), CD19 (1.0%), CD22 (26%), CD79a (0.3%), anti-terminal deoxynucleotidyl transferase (0.4%), CD38 (65%), CD3 (1.2%), CD7 (2.2%), CD3cy (6%), CD13 (78.9%), CD14 (3%), CD15 (17.4%), CD33 (99.2%), CD117 (25%), human leukocyte antigen/DR (20%), CD56 (7.5%), and CD34 (23.1%).
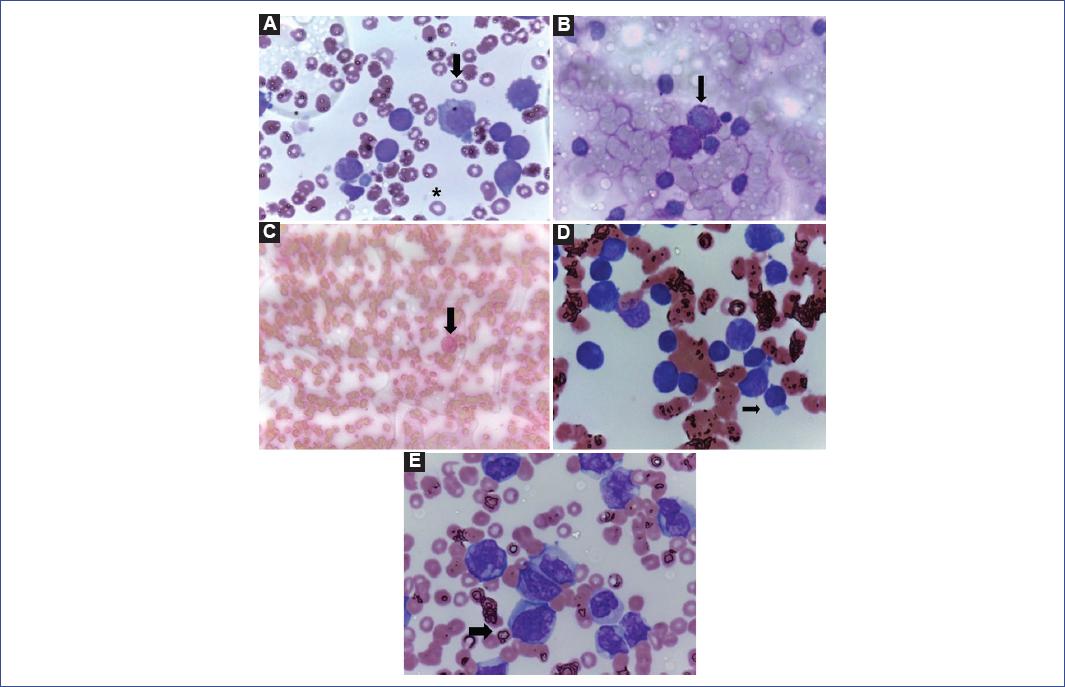
Figure 1 A: Bone marrow at diagnosis, which is characterized by megaloblastic cells with features of a microlobular shape, multinucleated appearance, dispersed chromatin, and a large nucleolus with cytoplasmic vacuoles (arrow). The asterisk shows an abnormal erythrocyte around 6-8 µm to compare cell dimensions. B: Positive PAS staining on bone marrow blast cells at diagnosis (arrow). C: Negative Sudan-black staining on bone marrow at diagnosis (arrows). D: Bone marrow at relapse (arrow). E: Peripheral blood at relapse. All samples were imaged at 100X.
We performed a reverse transcription-polymerase chain reaction (RT-PCR) screening of the BM and used an in vitro diagnostic test (HemaVision® test) for fast and sensitive detection of chromosomal translocations associated with leukemia. The same kit contains an internal quality control performed each time the test which was conducted. A positive FUS-ERG fusion transcript t(16;21)(p11;q22) was detected.
The cerebral spinal fluid analysis showed three lymphocytes per microliter and no blast cells. Cardiology assessment was as follows: left ventricular ejection fraction of 63% and preserved ventricular function. A Q-2019-40 skin biopsy was performed and indicated no infiltration of neoplastic cells in the skin. Follicular pustules with discrete infiltration by lymphocytes and histiocytes compatible with folliculitis were observed.
On January 12, 2019, the first cycle of chemotherapy was administered according to NOPHO-AML-93 (modified by changing thioguanine for mercaptopurine) as follows: cytarabine (200 mg/m2, intravenously), days 1-4; etoposide (100 mg/m2, intravenously), days 1-4; 6 MP (75 mg/m2, orally), days 1-4, and doxorubicin (75 mg/m2) on day 5.
After chemotherapy, the patient developed neutropenic colitis and active bleeding (hematochezia and epistaxis). Both complications were managed with the transfusion of blood products and antibiotic therapy (piperacillin/tazobactam and metronidazole).
On February 01, 2019, the BM examination revealed 7.5% blast cells, and RT-PCR still showed t(16;21)(p11;q22). Due to blast persistence, the patient was considered to have a poor response to the first cycle, and the second cycle was given as a second induction: cytarabine (100 mg/m2, intravenously on continuous infusion), days 1-5, and mitoxantrone (10 mg/m2, intravenously) on days 1-3. BM evaluation at 16 days post-chemotherapy reported morphological remission (2.5% blast cells), but t(16;21)(p11;q22) was still observed.
On March 16, 2019, a third induction cycle was started: cytarabine (2 g/m2, intravenously, twice a day), days 1-3, and etoposide (100 mg/m2, intravenously), days 2-5. After several infectious complications, the patient achieved morphological remission but showed a persistent molecular disease.
The BM transplantation team evaluated the patient, and after evaluation, no match was reported with related donors (two sisters were evaluated, one with 2/10 compatible haplotypes, and the other with 5/10 compatible haplotypes). The patient and her family requested not to continue with the international search process or other transplant options.
The patient received first, second, and third maintenance cycles on April 29, May 8, and June 24, 2019. The patient had multiple complications after the last cycle (neutropenia and fever with abdominal focus, proctitis, and neutropenic colitis). However, when the infectious processes were resolved, the patient continued to experience pancytopenia, so a BM examination was performed on September 13, 2019. The results documented a relapse to the cytomorphological BM of AML M6 on this date and the persistence of t(16;21)(p11;q22).
Information was given to the patient and parents. The process was quite difficult; the patient and her family requested not to continue with chemotherapy and decided to interrupt treatment and start with palliative care. The palliative care team was actively involved until the day of death. The patient was discharged home under palliative care supervision. She received supplemental oxygen, hydration solutions, and pain management. The date of death was documented 11 months after diagnosis.
Discussion
A case with a fatal outcome of a sporadic type of childhood leukemia has been described. In this case, t(16;21)(p11;q22) was presented de novo, unlike some reports where it presents as secondary leukemia.
According to previous studies, t(16;21) translocations in AML comprise t(16;21)(p11;q22) (FUS-ERG) as well as t(16;21) (q24;q22) (RUNX1-CBFA2T3). According to I-BFM, our data support that pediatric patients’ survival outcomes with AML with FUS-ERG are poor11.
Recent and isolated reports have analyzed some cases of pediatric AML with t(16;21)(p11;q22), revealing poor prognosis and suggesting that allogeneic hematopoietic stem cell transplantation could improve the prognosis and should be considered after complete remission. Some reports even consider the use of azacitidine13. However, given the infrequency of this subgroup, international collaboration is required for the prognostic relevance of rare genetic aberrations in AML.
We believe that this report is useful for the scientific and pediatric hemato-oncology community since it enriches the experience that will allow the classification of these patients into clinical and risk groups. According to our experience, it also adds consistency to the international experience. Thanks to the experience of a 21-year cohort, it has been possible to analyze a single series of pediatric patients. Furthermore, it is important not only to identify the molecular alteration but also to adequately characterize which of two transcripts that have been reported is present. Finally, a collaboration between different hospitals could help create cooperative groups between Latin American countries and other regions, to foster unity between professionals, and give consistency to the internationally reported knowledge.











 nueva página del texto (beta)
nueva página del texto (beta)


