Introduction
Pediatric thyroid carcinoma remains as a rare diagnosis despite approximately 1% annual rate increase. Overall, pediatric thyroid carcinoma demonstrates an excellent prognosis when identified early and treated adequately. Thyroid resection combined with the selective use of radioactive iodine (RAI) ablation is a safe and effective treatment for recurrent papillary thyroid cancer (PTC) in children. However, lymph node extension is a risk factor for recurrence (26-47%)1,2.
Growth hormone deficiency (GHD) is the main endocrinopathy in pediatric cancer survivors3,4. This disorder is observed in some types of pediatric cancer, such as acute lymphoblastic leukemia and brain tumors, and it is mainly attributable to the disease (type or location of tumor) or its treatment (surgery and radiotherapy)3. To the extent of our knowledge, the association between GHD and primary PTC or its treatment has not been yet reported.
GH and insulin-like growth factor-1 (IGF-1) affect tumor growthin vitroand some animal models5. Some studies have examined the relationship between GH treatment and cancer risk, but the current evidence is inconclusive3,5,6. In contrast, evidence exists regarding the benefits of GH treatment in childhood cancer survivors with GHD4,7,8.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children9. NAFLD has been associated with hypothyroidism, adrenal insufficiency, hypogonadotropic hypogonadism, and GHD10. Furthermore, cases of NAFLD associated with GHD with a satisfactory response to GH treatment have been reported7,11,12. However, no cases of NAFLD have been reported in patients with GHD with a history of primary PTC.
Case report
A 10-year-old male patient was referred to the endocrinology consultation due to short stature. He was born at full term by elective cesarean section after an uncomplicated first gestation with a birth weight of 2950 kg, height 49 cm, and Apgar 7/9. No history of blood transfusion or drug abuse was reported. He has normal neurocognitive development and good school performance. His mother informed history of Hashimoto’s thyroiditis and a thyroid nodule resolved with hemithyroidectomy. Furthermore, a history of thyroid cancer in his maternal grandmother was reported.
At the age of 5 years, a thyroid nodule was detected, and the biopsy indicated PTC with extension to the cervical nodes. He was treated with total thyroidectomy and required two doses of RAI ablation with 100 mCi due to the presence of functional thyroid tissue in the gland bed. The patient received levothyroxine 62.5 µg/day, which provided an adequate substitution.
The patient presented after 18 months of no follow-up for a growth evaluation. His mother reported low growth rate since he was 3 years old (before the diagnosis of thyroid cancer), for which the patient was worried. On physical examination, the patient appeared younger than his chronological age: height 116.5 cm (−3.51 standard deviation [SD]), weight 26 kg (−1.67 SD), and body mass index = 19.2 kg/m2 (+1.18 SD) according to child growth standards13(Fig. 1). No acanthosis or thyroid or cervical lymph nodes were detected. A large, firm liver was palpable, although his spleen was not palpable. His sexual development was in Tanner 1 stage.
Laboratory data revealed the following results: aspartate aminotransferase = 165 IU/l, alanine aminotransferase = 133 IU/l, gamma-glutamyl transpeptidase = 92 IU/l, lactic dehydrogenase = 318 mg/dl, alkaline phosphatase = 193 IU/l, total bilirubin = 0.57 mg/dl, direct bilirubin = 0.09 mg/dl, indirect bilirubin = 0.48 mg/dl, total cholesterol = 262 mg/dl, high-density lipoprotein cholesterol = 34.9 mg/dl, low-density lipoprotein cholesterol = 179.5 mg/dl, triglycerides = 238 mg/dl, white blood cell count = 4300/µl, hemoglobin = 12.8 g/dl, fasting glucose = 64 mg/dl, fasting insulin = 2.3 mIU/ml, and homeostatic model assessment for insulin resistance (IR) = 0.36. The results of serological testing for antibodies to antinuclear, antimitochondrial, anti-LKM (antiliver kidney microsome antibodies) and viral hepatitis (hepatitis A antibody, hepatitis B surface antigen, hepatitis B surface antibody, hepatitis B core antibody, hepatitis C virus antibody, and hepatitis C virus-RNA) were all negative. His bone age was evaluated as 8.5 years using the Greulich-Pyle atlas for a chronological age of 10 years. Other laboratory results included thyroid-stimulating hormone (TSH) = 0.86 mIU/l (normal range 0.8-6.9), triiodothyronine = 1.44 ng/ml (normal range 0.8-2.1), thyroxine = 7.98 µg/dl (normal range 5.5-12.8), free thyroxine = 0.95 ng/dl (normal range 0.65-1.9), thyroglobulin = 7.71 ng/ml (normal range 12-113), antithyroglobulin = 5.3 IU/ml (normal range <1.0), IGF-1 = 65.4 ng/ml (−2.66 SD), adrenocorticotropic hormone = 35.2 pg/ml (normal range 6-48), morning cortisol = 23.91 µg/dl (normal range 3-25), and 25-hydroxyvitamin D = 7.8 ng/ml (normal range 30-100).
Hepatic ultrasound revealed hepatomegaly with fat deposition suggestive of moderate hepatic steatosis (Fig. 2) without splenomegaly. The family did not agree to perform a liver biopsy. No abnormal brain findings were shown by magnetic resonance imaging (Fig. 3). Thyroid gammagram with 131I and thyrotropin alpha stimulation (TSH = 43.26 mIU/l) was considered within the normal limits, and no data suggested residual thyroid tissue.

Figure 2 Hepatic ultrasound. The echogenicity of the liver was higher than that of the renal cortex, with intrahepatic vessels not well depicted.

Figure 3 Magnetic resonance imaging in the pituitary region topography with no evidence of lesions occupying the space and glands with normal signal intensity.
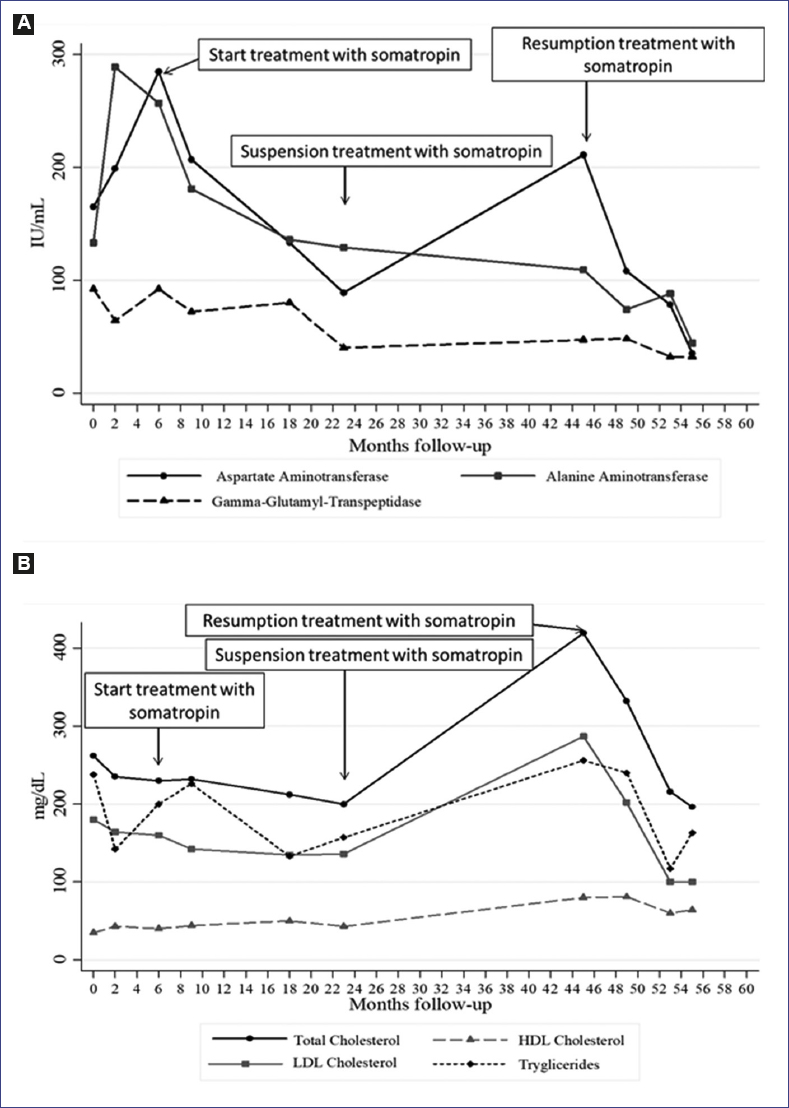
The treatment with levothyroxine at a dose of 62.5 µg/day, supplementation with Vitamin D and nutritional support continued. No significant improvement in growth was observed (Fig. 1), and transaminases continued to increase (Fig. 4). Due to liver alterations, lower IGF-1 levels in a second sample (30 ng/ml; −3.93 SDS), very short stature and the fact that the last screening did not show residual thyroid tissue, the mother and the patient were strongly advised to decide for GH treatment. The risks of the treatment were explained, mainly those related to the history of TPC with cervical node extension. The treatment started with somatotropin at a dose of 0.25 mg/kg/week and continued with levothyroxine.
After 17 months of somatotropin treatment, the patient reported more energy, improved self-esteem, and better general conditions. Furthermore, hepatomegaly reduction with a marked decrease of transaminases was observed (Fig. 4A), as well as an improved lipid profile (Fig. 4B) and a significant growth improvement (Fig. 1). The patient could not afford the somatotropin treatment any longer for which he spent 21 months without medical supervision. However, he continued the levothyroxine treatment, nutritional support, and physical activity.
Later, he turned to the service reporting fatigue and a low growth velocity rate (Fig. 2). Laboratory tests showed abnormal liver function and lipid profile (Fig. 4). Due to the evolution of the patient, treatment with somatotropin was resumed.
IGF-1 levels were found within the normal range during the treatment with GH (152 ng/ml, −1.3 SDS at age 12 and 210.7 ng/ml, −0.49 SDS at age 13). At present, the patient reports good general condition: growth rate is recovering, the puberty started (Tanner 2), and transaminases and lipid profile have reached normal values (Fig. 4) with the resolution of hepatic steatosis on ultrasound (Fig. 5). At present, at 14 years of age, no recurrence of thyroid cancer has appeared 9 years after diagnosis and 4 years after the start of GH therapy. The last thyroid gammagram with 131I was considered within the standard limits and no data suggestive of residual thyroid tissue and undetectable TSH-stimulated thyroglobulin (with negative Ab antithyroglobulin). At present, the patient continues with levothyroxine treatment, Vitamin D and GH and undergoes routine examinations for cancer follow-up and hormonal replacement.
Discussion
To the extent of our knowledge, this is the first case report describing a pediatric survivor of primary PTC with GHD that contributed to the development of fatty liver disease, which improved after GH replacement therapy. There was no cancer recurrence for 4 years of follow-up.
Auxological criteria and the low levels of IGF-1 (−2.66 SDS at age 10 and −3.93 SDS at age 11) support the diagnosis of GHD in this patient. However, the lack of GH generation tests would be a limitation for the diagnostic approach in this case. Due to the pulsatile GH secretion and minimum GH concentration between pulses, the random serum GH concentration is of no clinical utility. Instead, GH provocation tests are considered the basis of GHD diagnosis, as no true gold standard exists14-17. IGF-1 has been suggested to have a high specificity but relatively poor sensitivity for GHD diagnosis15,18. Guzzetti et al. reported a cutoff point of −0.5 SDS for sensitivity at 95% and −2.9 SDS for specificity at 95%19, which means that a standard value of IGF-1 does not exclude GHD, but the lowest levels, as in this patient, are highly predictive of GHD. Patients with GH resistance or primary IGF-1 deficiency also show the smallest values of IGF-1. In these cases, the response with GH is not satisfactory20, which contrasts with the response of the patient of the present report.
Late-onset endocrine abnormalities in survivors of childhood cancer can include GHD and other hormone defects3. The main clinical presentation in pediatric patients with GHD is short stature. However, the low growth rate can be induced by other conditions such as reduced nutritional intake, steroid administration, psychological dysfunction, and other abnormalities of the hypothalamic-pituitary glands (e.g., hypothyroidism and hypogonadism)4 that were excluded in this patient.
GHD is a consequence of hypothalamic-pituitary-adrenal axis damage in pediatric cancer survivors due to a brain tumor, central nervous system surgery, or cranial radiotherapy21. However, PTC or RAI ablation treatment has not been associated with GHD. For that reason, no reports of thyroid cancer survivors treated with GH were found in literature. In this patient, GHD was considered as an independent entity that was not due to PTC. GHD might have started before the cancer diagnosis given that the patient showed a low growth rate since he was a toddler. However, further genetic analysis is required to find an explanation regarding the etiology of GHD.
GH replacement has been demonstrated to be an effective treatment for reducing growth impairment in survivors of childhood cancer with GHD8. Although there is some evidence that GH therapy does not increase the risk of malignancy, some authors have reported that a risk could exist, especially in terms of the development of subsequent neoplasms, and most authors cite the need for more additional evidence3,6,22,23.
Thyroid cancer has been reported as a secondary neoplasm, with a relative risk of 2.15-3.21 among GH-treated survivors23,24. Despite the development of secondary thyroid neoplasms in cancer survivors treated with GH, it cannot be concluded that GH induces carcinogenesis. It is well-known that radiation can induce long-term thyroid damage, including thyroid cancer, and over 90% of these patients have also received skull radiotherapy25.
Somatotropin can be used to treat GHD in childhood cancer survivors who are in remission, with the understanding that GH therapy might increase the risk of secondary neoplasms4,6,23. However, assessing recurrence or second malignancies during long-term follow-up is crucial24. Although pediatric thyroid carcinomas have an excellent prognosis if identified timely and managed adequately, children show a higher recurrence rate than adults (~47% for papillary thyroid carcinoma)2,26.
GHD is associated with multiple features of the metabolic syndrome, including IR, obesity, dyslipidemia, hypertension, and NAFLD12,27. Interestingly, the present case only developed hepatic fatty liver disease and never showed signs of overweight, obesity, or IR. On the one hand, these features could be related to another pathway of hepatic steatosis independent from obesity/IR. On the other hand, the development of these characteristics might be a question of time, as demonstrated in a retrospective study of patients with hypothalamic and pituitary dysfunction in which fasting blood glucose levels were elevated in approximately 5% of patients at the time of diagnosis and in 62% of patients later. However, in a retrospective study of adult hypopituitary patients with GHD, the prevalence of NAFLD was significantly increased independently of obesity28.
NAFLD is a complex clinical-pathological entity defined as an excessive accumulation of fat in the liver in the form of triglycerides, called steatosis (histological infiltration > 5% of hepatocytes)9,29. Unfortunately, the level of infiltration could not be confirmed with a biopsy in this case. NAFLD has been associated with an increased risk of all-cause and cancer-specific mortality among cancer survivors29. Although hypothyroidism appears to be an independent risk factor for NAFLD/NASH (non-alcoholic steatohepatitis), this patient always maintained adequate hormone replacement therapy. Growing evidence suggests that GHD and low IGF-1 levels may play significant roles in the development and progression of NAFLD, including steatohepatitis as an independent risk factor10,27,28.
Previously, other authors have reported NAFLD improvement with GH treatment in some cases7,10,11,30. A patient with GHD related to NAFLD who required liver transplantation and was treated successfully with GH replacement was reported11. Although no substantial evidence regarding the efficacy of GH for NAFLD in GHD (lack of clinical trials) was demonstrated in this patient, liver damage improved with the GH replacement therapy, and transaminases increased when the GH replacement therapy was suspended. Given the lack of effective diagnostic and treatment options currently available for children, this area certainly deserves further investigation31.
Therapy with GH or IGF-1 improves the degree of liver steatosis and fibrosis, upgrades mitochondrial function, and reduces oxidative stress, which results in histologic improvement in NAFLD32. There are some explanations for the mechanisms by which the GH/IGF-1 axis contributes to this improvement. Some studies in human and animal models have proposed that GH/IGF-1 regulates the expression of numerous genes such as Mup1 (major urinary protein 1), Selenbp2 (selenium-binding protein 2), Ccnd1 (cyclin D1), Socs2 (suppressor of cytokine signaling 2), Socs3 (suppressor of cytokine signaling 3), epidermal growth factor receptor (EGFR), IGF-1, NNMT (nicotinamide N-methyltransferase), IGFLS (insulin growth factor-like), P4AH1 (collagen proly 4 hydroxylase 1), SLC16A1 (solute carrier family 16 member 1), SRD5A1 (steroid 5 alpha-reductase 1), FADS1 (fatty acid desaturase 1), and AKR1B10 (aldo-keto reductase family 1 member B10). Furthermore, other serum markers of inflammation, such as TNFa (tumor necrosis factor alpha), TGFb (transforming growth factor beta), CCL3, and fibrotic markers such as Col1A2 (collagen type I alpha 2 chain) and Col3A1 (collagen type III alpha 1 chain) as well, which are involved in glucose and lipid metabolism32-37. In addition, the GH pathway has shown to play a significant role in liver regeneration through the control of EGFR activation. GH/EGFR pathway downregulation is a general mechanism responsible for liver regeneration deficiency associated with steatosis38.
GH treatment could benefit growth and metabolism in cancer childhood survivors3,6,22,23. In the present case, the decision to start GH was controversial. Despite the theoretical risk of cancer recurrence, it was decided to begin the treatment in this patient to improve his growth rate, but mainly to limit the damage to the liver. Although therapy with GH can be safe, a close long-term follow-up is necessary for childhood cancer survivors. Concerns about the potential to increase the risk of developing cancer (de novo, recurrence, or secondary neoplasms) continue. Both his family and the medical team recognized the potential risks of GH treatment, for which is necessary to persist with long-term surveillance of GH therapy and the current recommendations for the follow-up of PTC in children1,2,24.
The patient should be reevaluated for GHD on completion of growth with a pharmacological stimulus (e.g., insulin, glucagon, and ghrelin)16,39. If GHD diagnosis is confirmed, GH replacement therapy in adulthood will continue to be controversial. On the one hand, the theoretical risk of recurrence of cancer with GH and, on the other hand, the deleterious effects on bone mineral density, lipid profile and liver function tests without GH treatment40.
As an overall conclusion, GH therapy could be a good therapeutic option for pediatric cancer survivors to address impaired growth and fatty liver disease. However, additional medical evidence based on clinical trials is necessary to evaluate benefits versus risks. Surveillance is essential for all individuals who are treated with GH, particularly those with an underlying predisposition for developing cancer.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.











 nueva página del texto (beta)
nueva página del texto (beta)