Introduction
Mutations affecting the ATP1A3 gene (OMIM 182350) have been associated with a group of related neurological phenotypes including four well-described syndromes: rapid-onset dystonia parkinsonism (RDP), alternating hemiplegia of childhood type 2 (AHC) (OMIM 614820), cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss syndrome (CAPOS)1, and relapsing encephalopathy with cerebellar ataxia (RECA). Defects in ATP1A3 are also associated with less specific phenotypes such as seizures, dystonia, ataxia, and psychiatric conditions2,3.
AHC is an autosomal dominant disease characterized by neurological symptoms, which include episodes of alternating hemiplegia, dystonia, abnormal ocular movements, autonomic dysfunction, and epileptic seizures, and impairments in speech, fine and gross motor skills. The estimated incidence of AHC is 1 in 1,000,0003,4. It is suggested that AHC type 2 and RDP are allelic disorders and comprise a single spectrum of a disorder associated with mutations in ATP1A35. There are 67 pathogenic mutations in ATP1A3 gene reported in ClinVar database6 with variable neurological phenotypes.
The symptoms of AHC start before 18 months of age and have a marked variability between different individuals who recover from the episodes of hemiplegia during sleep4. AHC can be diagnosed on a clinical basis, but the verification of a mutation in ATP1A2 (OMIM 104290) or ATP1A3 genes are necessary to confirm a clinical diagnosis3.
In this case, we report a Mexican patient with a movement disorder, who was referred to us due to hyperammonemia and a possible organic acidemia. We performed next-generation sequencing of a panel of genes associated with Mendelian diseases, which led to a final diagnosis of AHC.
Case report
We report a 5-year-old girl (individual II2) born in Los Mochis, Sinaloa, located at the northwest of Mexico. The parents are a young couple and were under 30 years at patient conception. The mother (individual I2) is affected by non-celiac gluten sensitivity and polycystic ovary syndrome; the father (individual I1) has periodical occupational exposure to pesticides without other medically relevant information. Proband has a healthy 7-year-old brother (individual II1, Fig. 1A).
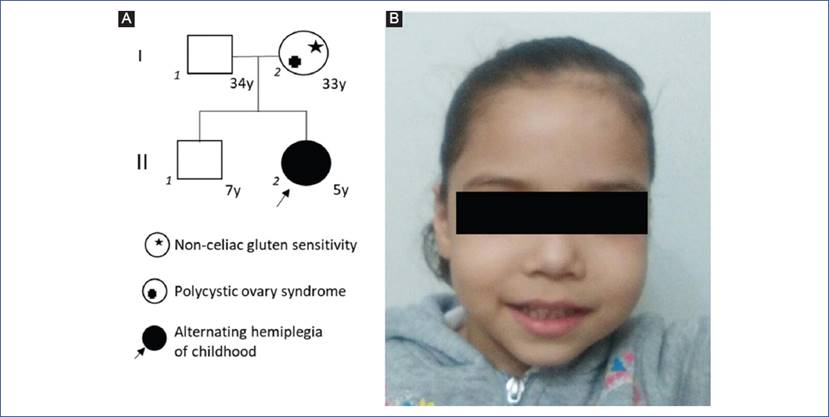
Figure 1 (A) Family pedigree. Proband (II2) is marked by an arrow. In this family, a simple case cannot support a specific pattern of inheritance of the neurological condition; molecular diagnosis confirmed a de novo presentation. (B) Patient facial phenotype with a long face, high forehead, horizontal eyebrows, full cheeks, long philtrum, slightly downturned mouth with an everted lower lip. Parents signed written consent for the publication of this image.
After a full-term pregnancy (39 weeks of gestation), without complications detected in the prenatal period or during delivery, the patient was born with normal height, weight, and Apgar score. No newborn screening of any type was performed. The patient showed developmental delay during her first year of life: she achieved cephalic control at 8 months, sitting at 12 months, and fluent language at 17 months. Currently, she requires support to walk. Her movement disorder started with an episode of alternating temporary hemiplegia with abnormal eye movement lasting several hours at the age of 8 months-misdiagnosed as epilepsy and initiated antiepileptic drugs. After this event, she continued to have episodes of hemiplegia every month, which increased with time in length, severity, and frequency, reaching a maximum of 4-5 events per week. These episodes were exacerbated by excitation and food with high sugar content.
At the age of 6 months, a transfontanelar ultrasound was reported as normal. At the age of 2 years, a normal MRI was reported. At this age, basic blood chemistry and hepatic profile were within normal range. Ammonia levels measured by independent labs ranged from 150 to 172 µmol/l (normal values 31-123 µmol/l) which were interpreted as a marker of a neurometabolic genetic disorder.
The patient was referred to the Genetics clinic by a neurologist after a poor response to anti-epileptic drugs and worsening of neurological symptoms, including dystonia, dysarthria, ataxia, and choreoathetosis with general rigidity.
Upon examination, the patient exhibited subtle dysmorphic facial features, namely, a long face with a high forehead, horizontal eyebrows, full cheeks a long philtrum and a slightly downturned mouth with an everted lower lip (Fig. 1B). After the initial clinical evaluation, plasma amino acids, acylcarnitines, and organic acids in urine were analyzed by mass spectrometry. Results were CH05: 0.05 nmol/ml (normal value for age < 0.12 nmol/ml), plasma amino acids, acylcarnitine, and organic acids in urine under normal limits. After ruling out the diagnosis of a metabolic disorder based on this biochemical test results, we decided to perform a next-generation sequencing panel with targeted DNA sequencing approach for Mendelian disorders, which captures exons of 4,813 genes previously associated to genetic disease. Sequencing was performed with a Miseq sequencer (Illumina) using Trusight One Panel (FC-141-1006, Illumina) panel under manufacturer protocols. Sequence alignment was performed using BWA (version 0.7.9a-isis-1.0.0), and variants were called with GATK 1.6 (v1.6-23-gf0210b3) using Miseq Reporter v.2.6 and downstream variant analysis with ANNOVAR7 using web version (http://wannovar.wglab.org/).
No variants were found in GCDH, ETFDH, ETFA, ETFB or SUGCT genes, which, when mutated, produce glutaric aciduria. A heterozygous missense variant c.2839G>A (p.Gly947Arg) located at exon 21 of ATP1A3 gene (NM_152296.4) was identified (Fig. 2A). This variant, rs398122887, has been independently reported as pathogenic by clinical laboratories in Clinvar database6 and literature8. The variant was confirmed by bi-directionally Sanger sequencing using BigDye® Terminator Cycle chemistry in an Applied Biosystems 3130 Genetic Analyzer (Fig. 2). The p.G947R was not present in parental DNAs (Figs. 2B and 2C) supporting a de novo origin.
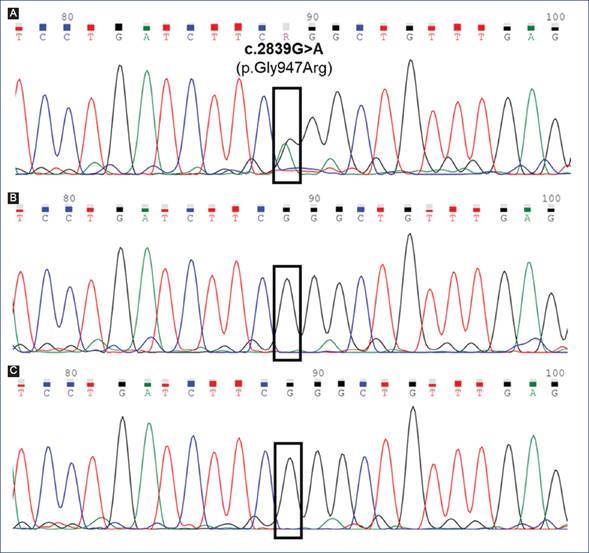
Figure 2 Partial electropherograms show the sequence of exon 21 of the ATP1A3 gene obtained by Sanger sequencing. A. Proband, the black square indicates the localization of the heterozygous pathogenic missense variant NM_152296.4:c.2839G>A (p.Gly947Arg) (rs398122887). Mother (B) and father (C) were homozygous for reference allele G at the c.2839 position, supporting a de novo origin of the missense variant.
After the confirmation of AHC, the patient started treatment with flunarizine 2.5 mg daily and control of triggering agents of hemiplegia. The patient improved from a frequency of events from 4-5 weekly (16-20 per month) to 1-2 monthly and experienced a better quality of life.
Discussion
In this patient, slightly increased levels of ammonia were misinterpreted for years by pediatricians and neurologists as an inborn error of metabolism. However, after a careful clinical exam, it was clear that the patient had no metabolic crisis and that the hemiplegia episodes were not related with protein consumption, as it is expected for inborn error of metabolism of lipids or amino acids. Using a massively parallel sequencing approach, we were able to diagnose the patient with AHC. This is likely the first patient diagnosed with AHC in Latin America. The variant p.Gly947Arg has been reported in patients of different ethnicities and their clinical presentation matches with that our patient4. Interestingly, the finding of recurrent de novo variants at position 947 of ATP1A3 suggests a mutational hotspot in this gene. The p.Gly947Arg pathogenic variant is associated with a better prognosis of AHC type 2 according to other reports in the medical literature4. This variant produces impaired Na/K-ATPase transport activity, which results in abnormal ion gradients and depolarization membrane potential. It is considered as evidence that the partial loss of function of Na/K-ATPase is responsible for AHC9.
From a clinical perspective, it is important for case reports of patients of different ethnicities with ultra-rare syndromes, such as AHC to elaborate a detailed genotype-phenotype correlation, because of the highly variable clinical expressivity of ion channel diseases. Additionally, an identical ATP1A3 mutation may result in distinct phenotypes. Indeed, the ATP1A3 variant, c.2767G>A (p.Asp923Asn), has been observed to cause both RDP and AHC1,10.
AHC is a poorly recognized disorder, and thus it is important for pediatricians and neurologists to be aware of this condition when performing clinical evaluations of children with seizure-like episodes or movement disorders. Diagnosis is challenging because hemiplegia episodes, which may occur only at home, may be misinterpreted by parents as an epileptic crisis. Clinicians should be aware of the combination of hemiplagia/hemiparesis without epilepsy. Some subtle dysmorphic facial features are emerging as a recognizable phenotype in AHC11; however, we acknowledge that expertise in dysmorphology is required tor recognize the AHC facial phenotype. This patient has the facial features (Fig. 1B) reported in other AHC patients11.
The patient has improved with flunarizine treatment, which illustrates the importance of an accurate and timely diagnosis in patients with rare disorders. The diagnosis of AHC also helped the family to receive an accurate risk of recurrence for future pregnancies.
In conclusion, massively parallel sequencing can modify our approach to diagnose ultra-rare disorders in neurology and metabolic medicine. In this patient, usage of a next generation sequencing (NGS) panel enabled a shorter and cost-effective pathway to the diagnosis, compared with the traditional method of gene-by-gene testing. This has led to a significant improvement in the quality of life of the child and the family. We hope that NGS testing will be more accessible for Latin-American public health systems in the near future.











 nueva página del texto (beta)
nueva página del texto (beta)


