1. Introduction
Urinary tract infections (UTIs) are diseases caused primarily by bacteria, which migrate from the perineal region and proliferate in the urethra, bladder, ureters or kidneys.1 However, migration may take place by hematogenous dissemination, direct contamination in medical procedures or traumas, or even through the bacterial translocation from the rectum to the urethra.2 The risk of urinary tract infection in the first ten years of life is 1% for males and 3% for females.3 Principal risk factors include constipation, family and personal history of urinary tract infections, improper hygiene, failure to thrive, and anorexia.4 The recurrence of UTIs is common (15-20%), especially in the first year after an initial occurrence and the risk increases with the number of prior occurrences (up to 60-75% of the cases with three or more occurrences).5 The absence of a diagnosis upon the occurrence of a urinary tract infection or its poor management may favor irreversible kidney damage.6
The diagnosis of UTIs with safety and reliability is essential to any treatment plan and its subsequent follow-up, taking into consideration that a poor diagnosis may have repercussions on the future life of the child and cause a burden from unnecessary examinations in the child, his family, and the health system.7,8 The estimated direct cost of the treatment that UTIs may require is approximately 6 billion dollars annually on health services worldwide.9
Appropriate clinical assessment of pediatric patients would be of great assistance in controlling infant morbidities and avoiding the possible complications of recurrent infections.10
The purpose of this study was to determine the pattern of antimicrobial resistance presented by Escherichia coli in pediatric patients admitted to the Department of Pediatrics, Hospital de Especialidades de las Fuerzas Armadas No. 1 (HE-1), and to identify the main clinical and laboratory manifestations of the UITs. These results could provide general practitioners and pediatricians with statistical data for an accurate diagnostic of UTIs.
2. Methods
We carried out an epidemiological, observational, descriptive case series study at the HE-1 in the city of Quito, Ecuador, from January to September 2015. We collected urine samples from 132 patients between 3 months and 14 years of age with clinical features suggestive of UTI. Seventy-three subjects were excluded since their respective cultures did not show E. coli growth, or because they were on antibiotic treatment before the sample collection. Neoplastic disease was also an exclusion criterion. The diagnosis of UTI was proposed by the medical history and physical examination of the patients. These data, together with a urine test (which was confirmed with a positive urine culture), suggested wherein a pathogen was isolated. Fifty-nine patients from the Department of Pediatrics participated in the study; these subjects were classified into two groups: hospitalization (H) and external consultation (EC). Based on the recommendations of the Instituto Nacional de Investigación en Salud Pública [National Health Research Institute] (INSP) and the regulations of HE-1, different antibiotic disks in the antibiogram were tested on each group. However, in antibiograms positive for extended spectrum beta-lactamase (ESBL) E. coli, all required disks were tested. The elemental and microscopic urine and Gram stain tests were performed on all samples. All blood samples were taken for a complete blood count (CBC), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) tests.
The Ethics Committee of the Universidad Central del Ecuador [Central University of Ecuador] supervised the study. All participants signed informed consents and were informed of the research applications that the results from their samples would have.
2.1. Urine sample collection
After washing the periurethral and perineal regions, urine samples were collected from each patient (under nursing supervision). Preferably, the sample was collected in the morning or with a 4-hour interval since the last micturition. In children younger than two years of age, the urine sample was taken through catheterization; in children older than two years of age, it was collected from a clean mid-stream urinary sample. The samples were kept and processed by the clinical and microbiological laboratories of the HE-1.
2.2. Blood sample collection
Peripheral blood samples were taken by venopunction before the initiation of antimicrobial therapy. Two samples per patient were taken in red and lilac cap tubes and were conserved and processed in the HE-1 Clinical Laboratory.
2.3. Urinalysis
The rapid urinary screening tests are urinary sediment, dipstick, and Gram stain. The dipstick test includes pH, proteins, glucose, ketones, blood, bilirubin, urobilinogen, nitrites, leukocyte esterase and urine density. With the urine sediment test, the following data were obtained: bacteria, nitrites, erythrocytes, hemoglobin, epithelial cells, and leukocytes.
2.4. Urine cultures
Urine cultures and antibiogram tests of the urine samples of all patients with a suspected UTI diagnosis were performed; these samples were processed according to the international standards from the Microbiology Laboratory of the HEP-1. Various antibiotic disks were tested in both groups of patients. All patients whose cultures contained the minimum quantity of bacteria were included in the study. The results obtained were recorded in the hospital software.
Methods used for the antibiogram were both automated (Vitek compact 2) and disk diffusion (Kirby-Bauer) by the NCCLI 2015, both for the minimum inhibitory concentration (MIC) and for the diameter of the inhibition halo.
HE-1 Microbiology Laboratory determines the presence of ESBL strains using various methods: identifying the species, testing broad panels of antibiotics and investigating rare phenotypes.
The double disk diffusion technique is based on double disk synergy. Briefly, a Müller-Hinton agar plate is inoculated with a bacterial suspension; then, cephalosporin and β-lactamase disks are placed at a certain distance (30 mm or 20 mm for more sensitivity) from the clavulanic acid. If there is an increase in the space between the inhibition halos of some of the antibiotics and the disk with the β-lactamase inhibitor, an ESBL is deemed to exist. In our case, the presence of ESBL was confirmed when the inhibition halo was equal to 5 mm on cephalosporin alone.
For the E-test technique, paper strips impregnated with antibiotics were used. One-half contained cephalosporin in decreasing concentrations, and the other half contained cephalosporin in decreasing concentrations with clavulanic acid in a fixed concentration of 2 g. The synergy of these antibiotics was considered positive when the MIC decreased in two or more dilutions.
2.5. Analysis of clinical histories
The clinical history number and UTI diagnosis were registered using the logbooks from the Pediatrics Department. Each clinical history was reviewed, and the software from the HE-1 was used to record the data from the anamnesis of each patient. The clinical signs or symptoms were registered to detect the most common clinical manifestations.
The collection of the microbiological and antibiogram data of each patient was performed with the assistance of the Microbiology Laboratory personnel.
2.6. Statistical analysis
Data were recorded and analyzed. Analyses were performed using SPSS software and Excel, both in their latest versions for Windows 10. The qualitative variables were compared to each other using a Pearson correlation. The comparison between the quantitative and qualitative variables was carried out with an odds ratio (OR). We calculated the risk association between clinical findings and the resistance pattern of E. coli.
3. Results
3.1. Clinical and laboratory findings
Clinical and laboratory data from patients were registered (Tables 1 and 2). Females presented UTIs with a higher frequency (H: 79.41%, EC: 92%), under five years of age (H: 4.23, EC: 3.35) in both groups hospitalization (H) and external consultation (EC). The most common urinary and non-urinary clinical manifestations observed were fever (H: 76.5%, EC: 88%), congestive oropharynx (H: 38.2%,EC: 40%), vomiting (H: 32.4%, EC: 32%), hyporexia (H: 20.6%, EC: 16%), abdominal pain (H: 20.6%, EC: 28%), dysuria (H: 14.7%, EC: 32%), and foul-smelling urine (H: 14.7%, EC: 12%). It is important to take into consideration that in the EC group, a big percentage of patients showed irritability (32%). Table 3 shows the frequency of the number of clinical signs and alterations in the laboratory parameters that patients presented in the study.
Table 1 Clinical manifestations of patients with urinary tract infection.
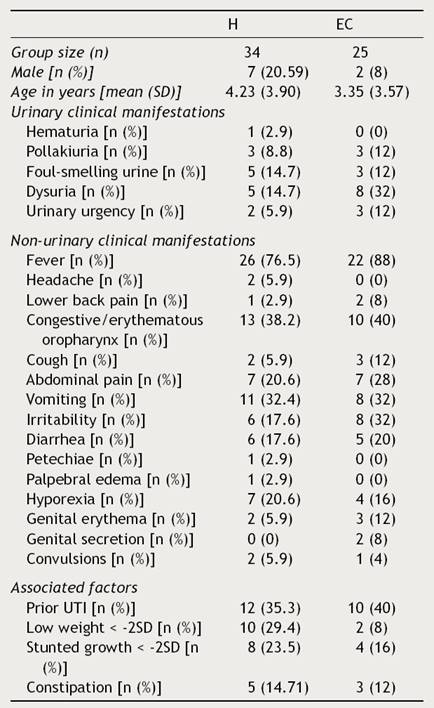
H, hospitalization; EC, external consultation; SD, standard deviation; UTI, urinary tract infection.
Table 2 Laboratory results from patients with urinary tract infections.
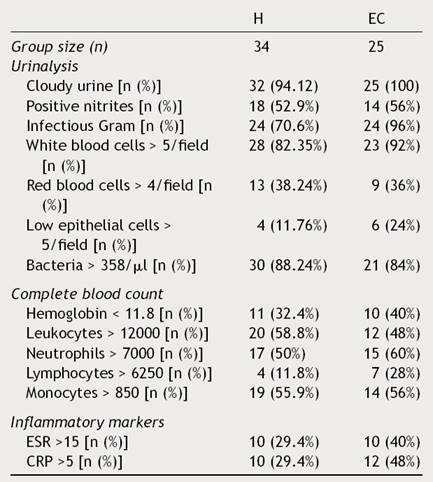
H, hospitalization; EC, external consultation; SD, standard deviation; ESR, erythrocyte sedimentation rate; CRP, C reactive protein.
3.2. Antimicrobial resistance of E. coli
Table 4 indicates the percentage of resistance of E. coli found in the antibiogram. Ten patients (16.95%) were found with ESBL E. coli UTIs. The antibiogram for third generation cephalosporins for patients from the EC group was only performed in cultures with a high probability of lactamase-producing strains. Five patients (EC: 20%) were corroborated with ESBL E. coli.
Table 4 Antimicrobial resistance of E. coli.
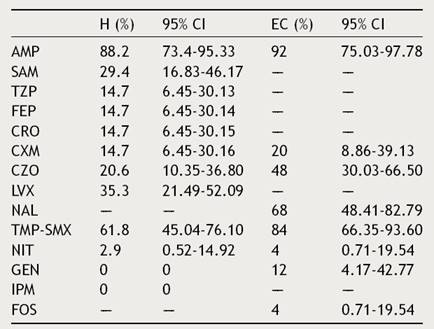
AMP, ampicillin; SAM, ampicillin/sulbactam; TZP, piperacillin/tazobactam; FEP, cefepime; CRO, ceftriaxone; CXM, cefuroxime; CZO, cefazolin; LVX, levofloxacin; NAL, nalidixic acid; TMP-SMX, trimethoprim/sulfamethoxazole; NIT, nitrofurantoin; GEN, gentamicin; IPM, imipenem; FOS, fosfomycin; H, hospitalization; EC, external consultation; CI, confidence interval.
3.3. Comparison of qualitative variables
The comparisons between data from the urinalysis and CBC and serological studies, and between weight and height by weight were non-statistically significant. However, a direct correlation between the number of leukocytes in the CBC and the quantity of CRP (r=0.39, r2=0.15, p<0.01), and between the percentage of lymphocytes from CBC and the number of lymphocytes in urine (r=0.29, r2=0.08, p<0.05) was found. Also, there was an inverse relationship between the number of bacteria in urine and the percentage of monocytes in CBC (r=-0.26, r2=0.01, p<0.05); however, these correlations were weak.10
The same correlation was found between leukocytes in CBC vs. CRP (r=0.9, r2=0.81, p<0.01) in patients who presented ESBL E. coli. In these patients, the quantity of CRP (r=0.67, r2=0.44, p<0.05) was greater at higher percentiles.
3.4. Temporal association between the risk of antimicrobial resistance and clinical findings
We compared clinical variables obtained from the patients with the antimicrobial resistance of E. coli in the H group, and we found 30 times more risk of carrying a strain resistant to aminopenicillin as a cause of UTI in patients who presented a previous cough (OR 31; CI 95% 1.02-94.0). On second generation cephalosporins, it was shown that patients with foul-smelling urine had 20 times more risk of having a UTI caused by a strain resistant to this family of antibiotics (OR 20.25; CI 95% 2.04–20.8). Similarly, it was found that patients who presented foul-smelling urine had a nine-time higher risk of being carriers of strains resistant to first generation cephalosporins isolated in urine cultures (OR 9.38; CI 95% 1.17-74.84). Another symptom that indicated an association with the resistance to antibiotics was diarrhea since it was observed that patients with this symptom had 13-times higher risk of being carriers of strains resistant to ureidopenicillins + β-lactamase inhibitor (BLI) (OR 13; CI 95% 1.51-111.8). Regarding fever, it was shown that in patients with this symptom the risk of presenting strains resistant to nitrofurans decreased by 24% (OR 0.76; CI 95% 0.63-0.92).
In the EC group, no significant association was found between resistance to antimicrobials and clinical symptoms.
4. Discussion
UTIs and respiratory tract infections are the most common pediatric bacterial diseases.11 It is estimated that between 8-10% of female and 2-3% of male patients will have a symptomatic UTI.5 The worldwide prevalence of pediatric UTIs is estimated at 5%, with an annual incidence of 3.1/1,000 girls (0-14 years) and 1.7/1,000 boys (0-14 years).12
After the neonatal period, the most common uropathogens are E. coli, Klebsiella, Proteus, Enterobacter, Citrobacter, Staphylococcus saprophyticus and Enterococcus,13 while group B streptococcal infections occur with greater frequency in newborns.2
In this study, we found that UTIs are clinically non-specific, which is consistent with the findings presented in other publications, even stating that making a UTI diagnosis based upon clinical manifestations has a 33% margin of error.14,15 Our data agree with other reports, where fever, abdominal pain, vomiting, dysuria, foul-smelling urine, and irritability are reported as frequent signs and symptoms of UTIs.4,16 In the urine sample study, we found that the presence of leukocytes, bacteria, nitrites and the Gram stain are the most common indicators of UTI and should be analyzed together. However, the absence of any of these does not exclude the diagnosis; therefore, urine culture is still the gold standard test.17,18
Ampicillin is the antibiotic for which we identified the most resistance, which is similar to other studies wherein resistance between 50-90% is reported. We have also found that there is resistance exceeding 20% for fluoroquinolones, first generation cephalosporins, trimethoprim/sulfamethoxazole and nalidixic acid, which is also consistent with other studies.19,20 Bacterial resistance to aminoglycosides, nitrofurantoin, and phosphomycin was low, which is consistent with the Ecuadorian National Bacterial Resistance Network (REDNARBEC).19,21 The percentage of ESBL E. coli is similar to other reports, between 5.5-33.3%.9,19,22 However, these values are high in comparison to a study reporting ESBL E. coli in 7.5% of the Pediatrics service.23 According to the antimicrobial resistance pattern found, the pharmacological groups that should be avoided as empirical therapy in this population are aminopenicillins, aminopenicillins+BLI, first generation cephalosporins, quinolones, fluoroquinolones, and folate pathway inhibitors.24
This study has important limitations that should be considered; one of them is the reduced number of patients. Based on this, it is necessary to increase the number of patients. Another limitation is the fact that we only considered data from our hospital for the analysis. It is important to use data from other health institutions for further investigations and to make a consensus for a better understanding of UTIs in our population. Also, it would be interesting to analyze the association between clinical or laboratory data and the time of hospitalization of the patients, or the association of ESBL E.coli with hospitalization length of stay.
In conclusion, and according to our findings and the increasing incidence of infections by broad spectrum beta-lactamase producing strains, we confirm the importance of not starting antibiotic therapy without a prior microbiological culture, especially in patients at risk of ESBL E. coli infection.25 However, initiation of empiric nitrofurantoin or phosphomycin therapy is a good option in the event of suspicion of cystitis, both in hospitalization and in outpatient services.26,27 When clinical manifestations suggesting gravity or upper urinary tract infection are present, therapy with aminoglycosides may be used, as they are also effective against ESBL E. coli.28,29











 nueva página del texto (beta)
nueva página del texto (beta)



