Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
Compartir
Boletín médico del Hospital Infantil de México
versión impresa ISSN 1665-1146
Bol. Med. Hosp. Infant. Mex. vol.70 no.2 México may./abr. 2013
RESEARCH ARTICLE
Expression of BART-5, BART-16 and BART-22, and NF-κB factor in classic Hodgkin's lymphoma in pediatric patients
Expresión de BART-5, BART -16 y BART -22, y del factor NF-κB en pacientes pediátricos con linfoma de Hodgkin clásico
Karina Liliana López-Facio,1 Pilar Eguía-Aguilar,1 Pedro Valencia-Mayoral,1 Mario Pérezpeña-Díazconti,1 Francisco Arenas-Huertero1
1 Laboratorio de Biología Molecular, Departamento de Patología, Hospital Infantil de México Federico Gómez, México, D.F., México
Corresponding author:
Dr. Francisco Arenas-Huertero
E-mail: farenashuertero@yahoo.com.mx
Received for publication: 02-05-13
Accepted for publication: 03-05-13
RESUMEN
Introducción. El linfoma de Hodgkin clásico es una neoplasia cuyo desarrollo se asocia con la presencia del virus del Epstein-Barr. No se conoce qué reguladores moleculares (como microRNAs del virus del Epstein-Barr) se expresan. Tampoco la asociación con factores proinflamatorios neoplásicos. El objetivo de este trabajo fue analizar la expresión de los microRNAs específicos del virus del Epstein-Barr, conocidos como BARTs (BART-5, BART-16 y BART-22), así como la expresión del factor NF-κB en pacientes pediátricos con linfoma de Hodgkin clásico.
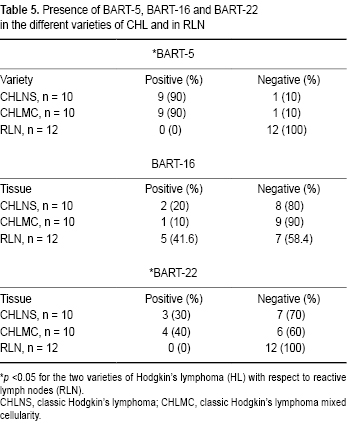
Métodos. Se seleccionaron 24 casos que cumplieron los criterios de inclusión. Entre las diferentes variedades, la esclerosis nodular y el de celularidad mixta fueron las más frecuentes.
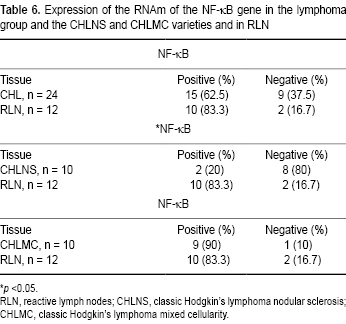
Resultados. Los resultados mostraron que la expresión del BART-5 fue la más frecuente (83%) en el linfoma de Hodgkin clásico. La expresión de BART-22 fue la segunda más frecuente, de 33.3% respecto a los testigos. En todos estos casos las diferencias fueron significativas respecto a los ganglios linfáticos reactivos (p <0.05). La expresión de NF-κB se encontró en 62.5% de los casos de linfoma de Hodgkin clásico, y se presentó en 83.3% de los ganglios linfáticos reactivos (p <0.05). La variedad de celularidad mixta lo expresó en 90% de los casos, lo que contrastó con 20% que presentó la variedad esclerosis nodular (p <0.05).
Conclusiones. Se puede concluir que la expresión del BART-5 fue la más frecuente en los casos de linfoma de Hodgkin clásico. También, que el factor NF-κB es un indicador importante de inflamación, y presentó mayor expresión en los ganglios linfáticos activos.
Palabras clave: virus Epstein-Barr, linfoma Hodgkin clásico, microRNAs, BARTs, NF-κB.
ABSTRACT
Background. Classic Hodgkin's lymphoma (CHL) is a neoplasm in which the presence of, or infection by, the Epstein-Barr virus (EBV) is associated with disease development. Two aspects of this condition are currently unknown: first, whether molecular regulators such as the microRNAs of EBV are expressed and second, if there is an association with inflammation-promoting, neoplastic factors in pediatric CHL. The aim of the present study was to use RT-PCR to analyze the expression of the specific microRNAs of EBV called BARTs, specifically BARTs-5, -16 and -22 and that of factor NF-κB, also using RT-PCR.
Methods. A total of 24 cases were selected after meeting the inclusion criteria, which involved different varieties of CHL including the nodular sclerosis (NS) and mixed cellularity (MC) types. These resulted in being the most common ones, each with a frequency of 41.6%.
Results. BART-5 was the one most frequently expressed in CHL, at 83.3%. BART-22 was the second most frequent, at 33.3%, compared to 0% in controls (reactive lymph nodes, RLN). In all cases, the differences compared to RLN were significant (p <0.05). Expression of NF-κB was found in 62.5% of CHL cases and was present in 83.3% of RLN (p <0.05). The MC type expressed it in 90% of cases, compared to only 20% for the NS variety (p <0.05).
Conclusions. BART-5 was the one most frequently expressed in CHL cases. NF-κB factor is an important indicator of inflammation most often expressed in RLN.
Key words: Epstein-Barr virus, Hodgkin's lymphoma, microRNAs, BARTs, NF-κB.
Introduction
Classic Hodgkin's lymphoma (CHL) is a monoclonal lymphoid neoplasm composed of mononuclear Hodgkin and multinucleated Reed-Sternberg cells found in a context of mixed inflammatory that includes lymphocytes, eosinophils, plasma cells, neutrophils, histiocytes and fibroblasts.1 Lymphomas are the third most common malignant neoplasm in children after leukemias and tumors of the central nervous system.2 In nonindustrialized countries such as Mexico, this condition is less common in the general population, but more frequent in children.3 Data obtained from the Department of Biostatistics at the Hospital Infantil de México Federico Gómez (HIMFG) for the year 2009 show that HL had a morbidity rate of 35.7/1000 consultations, establishing it as the third most common malignant neoplasm in that year, with an index of 118 cases/1000 treated patients.4 One important property of this condition is the capacity of the B cells to evade apoptosis and thus survive and continue to proliferate. Several mechanisms that contribute to the survival of HL cells have been described, including the presence of, or infection by, the Epstein-Barr virus (EBV) and the role of the NF-κB protein. EBV is a herpes virus that infects almost 90% of the human population, primarily during infancy, but that persists throughout life. Although usually a benign infection, clinical cases do occur in the form of infectious mononucleosis, but this illness normally does not progress towards development of lymphoma. Moreover, some EBV carriers never manifest any clinical symptoms of the infection; although with time they may develop CHL. Cases of CHL associated with EBV infection occur in patients who never manifested clinical signs of the viral infection or demonstrated deficiencies in their antiviral immune activity. These facts clearly reveal the existence of different mechanisms of pathogenesis on the part of EBV that operate during clinical infection to induce the development of CHL and other neoplasms.5 EBV infections are also found in association with another type of neoplasm: Burkitt lymphoma. When this virus infects epithelial cells it predisposes them to the development of nasopharyngeal carcinoma and some types of gastric cancer.6 The first studies conducted with the aim of pinpointing the mechanisms of EBV infection revealed important differences in terms of how it infects an epithelial cell in vivo , a cell from the immune system like a B cell, and cultured cells in vitro. 7
Stage I of EBV infection is the one associated with the development of Burkitt lymphoma. It is characterized by expression of the protein related to LMP2A latency and the nuclear antigen 1 EBNA1, which is responsible for the viral replication of EBV. Some lytic events may occur during latency stage I.8 Stage II of EBV infection occurs during development of nasopharyngeal carcinoma when only some of the proteins that form part of the nuclear antigen-such as EBNA1 and LMP1 and LMP2A-are expressed. It has been suggested that this is how these viral proteins succeed in altering the signaling routes and mechanisms that lead to tumor development. Two non-codifying RNAs called EBERs (EBV-encoded RNAs) are transcribed and, once that is done, move towards terminal region 3´, which contains a Bam H1 A fragment called a BART (i.e., BamH1 A rightward transcripts) that can be detected by Northern-blot.9-11 BARTs are small, multi-edited RNAm (microRNAs) that were first reported in cases of nasopharyngeal carcinoma and later in individuals infected by EBV.12
In stage III, which is characterized by lymphoblastoid cells or lymphoproliferative disease, the 12 genes of latency are expressed, including the six nuclear proteins: EBNA-1 to -6; three membrane proteins (LMP-1, LMP-2A and LMP-2B); the BARTs; and EBER-1 and -2.9,13 However, these transcribed BARTs in lymphoblastoid cell lines are expressed at low levels that can only be detected by RT-PCR.14,15 The microRNAs of EBV are codified in three ''clusters'', plus a unique sequence known as BART-2. Of these three ''clusters'', two are in the BART region. ''Cluster'' 1 contains BARTs-1, -3, -4, -5, -6, 15, -16, and -17, whereas ''cluster'' 2 holds BARTs-7, -8, -9, -10, -11, -12, -13, -14, -18, -19, -20 and -22. ''Cluster'' 3 is located in the region called BAHRF1 (abbreviation of BamHI fragment H rightward open reading frame 1). It contains the microRNAs labeled BHRF1-1, BHRF1-2 and BHRF1-3. Both the BARTs and the cellular microRNAs are short sequences of a mature chain with 18-22 nucleotides that bond to their ''seed'' sequence at the non-translated 3´ end of more than one target RNAm, where they negatively regulate expression of the corresponding protein.16
The NF-κB transcription factor participates actively in the inflammatory response and is expressed constitutively in virtually all types of cancer.17 This family of factors has five members: RelA/p65, c-Rel, RelB, NF-κB1 (p50) and NF-κB2 (p52).18 Regulation of the NF-κB route is effectuated through an association with its inhibitor, I-κB, to NF-κB1 (p50) and RelA (p65), a trimer that normally exists in the cytoplasm. In response to diverse stimuli, I-κB undergoes tri-phosphorylation, a signal that indicates that it is to be broken down through the proteasome route to release the p50-p65 dimer, which was previously phosphorylated and translocated to the cell nucleus in order to activate the corresponding genes.19 The activated dimer may also cooperate with other transcription factors such as AP1, HIF-1α and STAT3.20 Mutations, primarily in p65, are more frequent in lymphoid neoplasms, and amplifications of p65 have even been described, mainly in B-cell lymphomas and, although less often, in T-cell lymphomas.21 Mutations or the deletion of I-κB have been described for cases of CHL where they propitiate the unregulated activity of the active dimer of p50-p65 that, in turn, activates transcription of the genes that participate in proliferation and anti-apoptosis. In most cases, mutations occur in NF-κB.22 The expression and high activity of NF-κB are characteristic of the chronic inflammation seen in certain types of cancers including CHL. It is for this reason that administering substances that help control inflammation produces a beneficial effect in most cases.23 The tumor-promoting effect of NF-κB consists of producing tumor-promoting cytokines through the interaction of NF-κB with factors such as STAT3 and AP1, which then activate the genes that participate in proliferation, survival, angiogenesis and metastasis.24
This study analyzed the expression of BARTs-5, -16 and -22 as well as the gene NF-κB1, in cases of CHL in Mexican children treated at the HIMFG.
Subjects and Methods
Characteristics of the cases
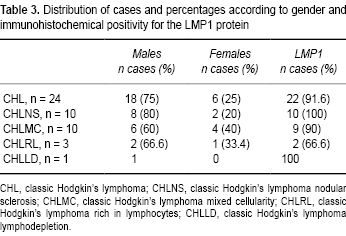
Cases of CHL were located in the records of surgical samples in the Pathology Department of the HIMFG from the period 2000-2008. For those years, a total of 89 cases of classic LH were found, but only 24 of these met the inclusion criteria and so were analyzed experimentally. They were classified histologically as follows: ten cases of the nodular sclerosis variety (CHLNS), ten with mixed cellularity (CHLMC), three with lymphodepletion (CHLLD) and one rich in lymphocytes (CHLRL). All H & E-stained slides from the selected cases were examined, and a representative sample was selected that had a fragment of viable tissue ~4 cm in diameter. The fragments were re-set in paraffin to order to obtain the sections to be used in the molecular studies. As tissue controls, the study used 12 biopsies from patients who had been diagnosed with reactive lymph nodes (RLN) but had no tumorous lesions.
Immunohistochemical staining for expression of the LMP1 protein
As part of the verification of the presence of EBV infection, immunostaining of the late membrane-1 antigen LMP-1 was performed using a primary anti-LMP1 (DAKO) antibody and revealed/developed using the universal system. The antigen was recovered by peroxidase reaction.
RNAm Extraction and Amplification of the BARTs and NF-κB
Two 10-µm sections in microtubes were obtained from the paraffin blocks with the re-set tumor samples from both LH cases and RLN. The paraffin was removed as follows: 1 ml of xylene was added and the mixture was shaken vigorously and then incubated for 5 min at 50°C before being centrifuged for 5 min at 10,000 rpm. This step was repeated with the same volume of xylene and then with absolute ethanol. Finally, the paraffin-free tissues were left to dry for 4 min at 40°C.
Next, the samples were digested in 100 ul of nuclease-free water with the addition of 4 µl of proteinase-K concentrate. They were incubated first for 15 min at 50°C and then for an additional 15 min at 80°C. Afterwards, they were frozen at -20°C for the later extraction of total RNAm. Total RNAm was obtained using the AMBION ''Recoverall Total Nucleic Acid Isolation'' kit following the manufacturer's instructions. Finally, the total RNAms were eluted in 20 µl of nuclease-free water. Synthesis of cDNA was performed using 100 ng of total RNAm in a final volume of 7.5 µl by means of the Fermentas ''First Strand cDNA Synthesis'' kit, with specific initiators for the BARTs and universal U6 microRNA as a control. To evaluate the expression of the BARTs, the loop-stem technique was used. The sequences of the initiators of the BARTs, U6, NF-κB, and GAPDH are shown in Table 1.
The next procedure involved sequence amplification by PCR using 1.5 µl of specific cDNA in a final volume of 50 µl under the following conditions: initial denaturation at 92°C for 5 min, then 30 cycles that consisted of denaturation at 92°C for 30 sec, alignment at 58°C for 30 sec, and elongation at 72°C for 30 sec. A final prolonged elongation at 72°C for 3 min ensued, with exit at 20°C. The size of the amplified products is shown in Table 2.
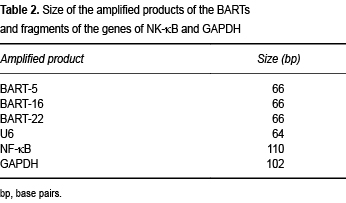
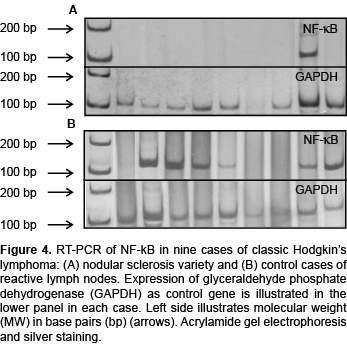
To reveal the amplification products, electrophoretic separations were conducted in polyacrylamide gels at 12%. Ten µl of the product from each amplification were charged by applying 90 V for 90 min. The amplification bands were revealed by staining with silver. The digital images thus obtained allowed us to consider the cases as positive for the presence of 66-base pair (bp) bands for mBARTs-5, -16 and -22, 64 bp for U6, 110 bp for NF-κB, and 102 bp for GAPDH.
Statistical Analysis
Because the expression of the BARTs and NF-κB was evaluated only as presence vs. absence, contingency tables were elaborated for each BART and NF-κB. Differences in the expression of each one were evaluated using Fisher's test; p <0.05 was considered statistically significant.
Results
Of the 89 total cases of CHL diagnosed in lymph nodes, the most frequent histological variety was CHLNS with 54 recorded cases (60%) followed by CHLMC, with 31 cases (35%), CHLLD with three cases (3.4%), and just one case of CHLRL (1.1%).
Of these cases, 65 (73%) were males and 24 (27%) were females. After the initial assessment, the 24 cases that met the inclusion criteria were selected for the study. Thus, the final study group was comprised of 18 cases (75%) of males and six (25%) of females, with an age range of 4-15 years and an average of 9 years (Table 3). Immunodetection of LMP1 was performed in all 24 cases of HL (Figure 1). Results show that 22/24 (91.6%) cases were positive for this marker that indicates EBV infection (Table 3).
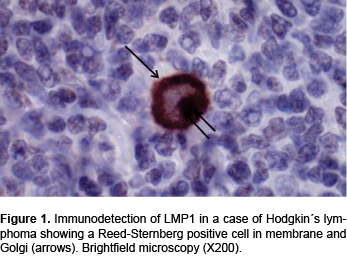
Later, RT-PCR was conducted for BARTs-5, -16 and -22 in all 24 cases. Results revealed the presence of all three BARTs. BART-5 was the most frequent and was found in 20/24 cases (83.3%) (Table 4, Figures 2A and 2B). The frequency comparison of BART-5 between cases of HL and controls resulted in being significant (p <0.05) as was also the case for the other two BARTs. The expression of BART-5 proved that 83.3% of cases were positive for EBV infection (Table 4) in contrast to the 91.6% positivity found for the presence of LMP1 (Figure 2). These differences were significant (p <0.05). BART-16 was the second most frequent one found in CHL cases, with 50% positive tests. It should be noted that BART-16 was present in more controls than cases (Table 4). These differences were also significant. On the basis of these results, it can be concluded that because BART-5 is the expression found most frequently in CHL cases, it is the best indicator of EBV infection in such cases.
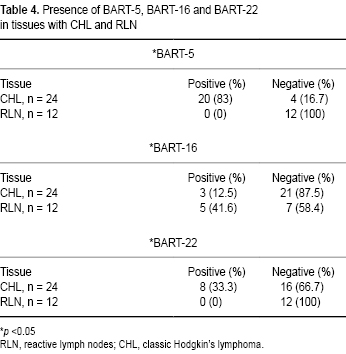
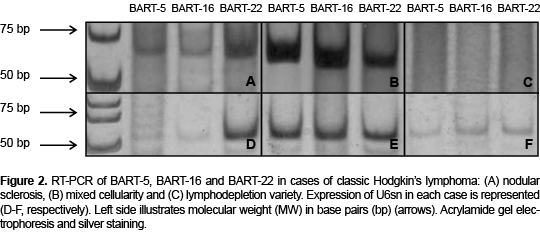
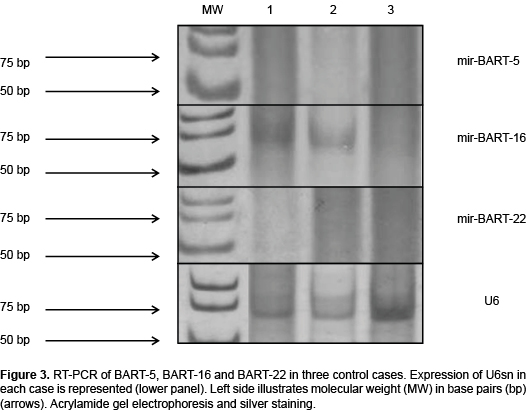
The next analysis was to detect the expression of each BART according to the variety of LH. This procedure involved only the nodular sclerosis (CHLNS) and mixed cellularity (CHLMC) types because there were ten cases of each, which allows the results to be compared to the 12 controls (Table 5). As Figures 2A and 2B show, these two types of LH were 90% positive for BART-5, a finding that contrasts with the nil result in controls (Figure 3). Differences were significant. The lymphodepletion variety of LH did not express BARTs (Figure 2C). Another notable finding is that three and four cases of CHLNS and CHLMC, respectively, were positive for BART-22, although no control cases were. These differences were also significant. On the basis of these results, it can be concluded that expression of BART-5 is characteristic of LH and is, at least, the one most frequently found in the nodular sclerosis and mixed cellularity histological varieties.
Finally, expression of the RNAm of factor NF-κB1 was analyzed separately from the BARTs. It participates in the onset/progression of CHL and is associated with inflammatory responses. Table 6 shows that the frequency of expression of NF-κB was higher in controls (83.3%) than in CHL cases (62.5%). This can be seen in Figure 4B, which compares the expression of this factor in controls with the nine cases of CHLNS in Figure 4A. Later, an analysis of the expression of NF-κB in the nodular sclerosis and mixed cellularity varieties was carried out. As can be seen, for the CHLNS variety only two cases (20%) were positive in contrast to the nine positive cases of CHLMC (90%). Compared to controls, these differences were significant (Table 6). On the basis of these results, it can be concluded that the association of factor NF-κB with important inflammatory responses explains why its expression was the one most often found in controls and the CHLMC variety.
Discussion
The objective of this study was to analyze the expression of BARTs-5, -16 and -22 and the expression of the NF-κB1 gene in samples of CHL from Mexican children treated at our Institute. The decision was made to analyze these specific BARTs because they form part of two of the most widely studied ''clusters'' and because the RNAm of the target cell genes has been validated.23 A second reason for this selection was that there are no reports of analyses of these factors in cases of pediatric CHL. Two BARTs from the first ''cluster'' were analyzed: BARTs-5 and -16. From the second ''cluster'', the study selected the expression of BART-22. It should also be noted that these BARTs have been reported as genes expressed exclusively in nasopharyngeal carcinomas that are EBV-positive,25 and our results demonstrate their expression in CHL cells infected with that virus. Thus, our findings support the idea that there is no specific pattern of cellular expression, at least in the BARTs, from these two ''clusters''. Moreover, our results demonstrate that expression of BARTs is associated with viral latency.26
Each one of these BARTs carries out negative regulation of some RNAm as mentioned above. These targets may be viral or cellular.26 Thus, for example, observation of the high expression of miR-BART-5 in the CHL cases studied implies that they are losing expression of the PUMA protein (p53 up-regulated modulator of apoptosis) required for the onset of apoptosis, which depends on the activity of the p53 protein. The RNAm of the PUMA protein has previously been validated as the target of BART-5.23 EBV infection is an event that damages DNA, but in order for it to achieve integration it is important that the PUMA protein be inhibited by the action of BART-5.27 In this study, 83.3% of the cases examined expressed this, so it is possible that inhibiting responses to DNA damage through negative regulation explains why BART-5 is expressed in more cases. On the other hand, some authors mention that another convenient condition is the overexpression of the LMP1 protein because this induces inhibition of the growth and sensitization to apoptosis due to stress or chemotherapy.28 Data concerning the action of LMP1 seem contradictory when we discover that BART-5 induces the breakdown of the PUMA protein and affects cell death; however, there are several routes of apoptosis induction-some intrinsic, others extrinsic-so it is important to determine which of these routes is activated preferentially in such cases. The expression of BART-5 in such a large number of cases suggests that it is an important target from the therapeutic perspective: i.e., for developing and applying an antagomiR-BART-5 that would induce the breakdown and expression of the PUMA protein. This suggestion obliges us to follow-up on these BART-5-positive patients and to compare them in terms of treatment response to those who test negative as well as to compare the survival curves of the two groups after considering a larger number of cases. The results of the present study demonstrate that BART-5 expression is also present in B-cell tumors such as pediatric CHL. This finding is similar to that reported by Edwards et al. who demonstrated the expression of these and other BARTs in lymphoid tumor cells and cases of gastric cancer.29
With respect to the expression of BART-16, which belongs to the same ''cluster'' as BART-5, we found that it was expressed in both samples: i.e., CHL and RLN cases, although at a high percentage only in the latter: 12.5% vs. 41.6%, respectively. What is important in these cases is that it has been proven that the target of this BART-16 is the RNAm of the LMP1 protein of EBV, i.e., it induces breakdown of the latter. What we have described is that LMP1 has oncogenic properties and produces signaling because it activates the TNF receptor. In 91.6% of cases, results were positive for LMP1 immunostaining. Of the cases that were positive for BART-16, none corresponded to those that were negative for LMP1. In all likelihood, this suggests that other mechanisms that regulate the expression of LMP1 may be operating in CHL tumor cells, ones distinct from those described for nasopharyngeal carcinoma.26 In these CHL cases, the presentation of antigens including LMP1 may be taking place, similar to findings reported for cells in nasopharyngeal carcinoma,6 although this has not yet been proven. With respect to the RLN group that expressed BART-16, the loss of LMP1 expression must be demonstrated because it may offer an advantage in terms of evading the immune system and, possibly, for tumor development through other routes. Worse yet, they may express LMP1, thus ensuring that cell transformation will take place along other routes.
In relation to the expression of BART-22, 33.3% of CHL cases were positive, but none of the RLN cases. The biological implication of this finding is that it validates the LMP2A protein as the target of BART-22. Because this protein is strongly immunogenic, this result suggests that in cases that are positive for BART-22 the tumor cells can evade the immune response and thus foster the process of tumor development and/or affect patients' response to treatment. Other functions of LMP2A include inhibiting the activity of the retrotranscriptase of telomerase by shortening the telomeres of the cell chromosomes, thus assuring their immortalization and transformation.6 It is not yet known whether these positive cases for BART-22 will manifest a poor response or show a more aggressive behavior. What is important is that this occurred in five of the eight cases of the CHL varieties with the poorest prognoses; i.e., MC (mixed cellularity), LD (lymphodepletion), and RL (rich in lymphocytes). This finding must be assessed by follow-up on these patients, increasing the number of cases, and comparing the survival curves of positive and negative cases, respectively, for BART-22 to LMP2A in these histological varieties because these factors may be related to prognosis.
In addition, infection with any kind of virus, including EBV, conditions the expression of a pattern of specific cellular microRNAs in which, in most cases, oncogenic activity is recognized.26 This could be another control point through the application of therapeutic antagomirs capable of negatively controlling the oncogenic response of the cell's onco-microRNA.
The expression of NF-κB is strongly related to the onset and maintenance of the inflammatory process in tumors in cases of both CHL and RLN because it is an important activator of inflammatory responses, especially in cases of the mixed cellularity variety where 9/10 (90%) were positive for expression of NF-κB. In cases of CHL and RLN, the NF-κB protein is produced as a result of the cytokines secreted by inflammatory cells such as TNF-α and interleukin 1β.20 Also, the processes of proliferation, angiogenesis, migration and metastasis, among others, can be activated and produced through the effect of the viral protein LMP1.26
It is important to expand this study to include a larger sample size and, above all, to focus on analyzing the expression of BART-2, which is found in only one region outside the three ''clusters'' and that negatively regulates expression of the polymerase of EBV to maintain it in the latent phase in cases of both CHL and RLN. This fact leads us to posit the possibility of assessing the negative regulation of BART-2 with therapeutic antagomirs that enable the transition to the lytic cycle of EBV because it seems to evade latency once inside the host. Finally, BARTs and cellular microRNAs are produced and secreted in the serum, a fact that suggests the possibility of studying these using less invasive techniques and recognizing them as serum markers of infection or of the response to treatment in EBV infections.
Acknowledgments
The authors thank the Division of Investigation of the Hospital Infantil de México Federico Gómez for the support provided for the present study through project no. HIM/2011/008, and the Scholarship Program of the Programa Universitario de Investigación en Salud at the Universidad Nacional Autónoma de México (PUIS-UNAM) (Karina Liliana López-Facio). We are also grateful to Consejo Nacional de Ciencia y Tecnología (CONACyT) and Sistema Nacional de Investigadores (SNI) (Francisco Arenas-Huertero).
REFERENCIAS
1. Stein H, Deisol G, Pileri S, Said J, Man R, Popema S, et al. Classical Hodgkin lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization Classification of Tumors. Pathology and Genetics: Tumours of Haematopoietic and Lymphoid Tissues; 2008. Lyon: IARC. pp. 326-329. [ Links ]
2. Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE. Nathan and Oski's Haemathology of Infancy and Childhood; 2003. Philadelphia: Saunders Elsevier. [ Links ]
3. Diehl V. Hodgkin's disease-from pathology specimen to cure. N Engl J Med 2007;357:1968-1971. [ Links ]
4. Departamento de Bioestadística y Archivo Clínico del Hospital Infantil de México Federico Gómez, 2010.
5. Mani H, Jaffe ES. Hodgkin lymphoma: an update on its biology with new insights into classification. Clin Lymphoma Myeloma 2009;9:206-216. doi: 10.3816/CLM.2009.n.042. [ Links ]
6. Lung RW, Tong JH, Sung YM, Leung PS, Ng DC, Chau SL, et al. Modulation of LMP2A expression by a newly identified Epstein-Barr virus-encoded microRNA miR-BART22. Neoplasia 2009;11:1174-1184. [ Links ]
7. Miller G, Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA 1973;70:190-194. [ Links ]
8. Amon W, Farrell PJ. Reactivation of Epstein-Barr virus from latency. Rev Med Virol 2005;15:149-156. [ Links ]
9. Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Fields BN, Knipe DM, Howley PM, eds. Fields Virology; 2001. Philadelphia: Lippincott-Williams and Wilkins. pp. 2511-2573. [ Links ]
10. Raab-Traub N, Hood R, Yang CS, Henry B II, Pagano JS. Epstein-Barr virus transcription in nasopharyngeal carcinoma. J Virol 1983;48:580-590. [ Links ]
11. Young L, Alfieri C, Hennessy K, Evans H, O'Hara C, Anderson KC, et al. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med 1989;321:1080-1085. [ Links ]
12. Cai X, Schäfer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, et al. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog 2006;2:e23. [ Links ]
13. Hitt MM, Allday MJ, Hara T, Karran L, Jones MD, Busson P, et al. EBV gene expression in an NPC-related tumor. EMBO J 1989;8:2639-2651. [ Links ]
14. Sadler RH, Raab-Traub N. Structural analyses of the Epstein-Barr virus BamHI A transcripts. J Virol 1995;69:1132-1141. [ Links ]
15. Karran L, Gao Y, Smith PR, Griffin BE. Expression of a family of complementary-strand transcripts in Epstein-Barr virus-infected cells. Proc Natl Acad Sci USA 1992;89:8058-8062. [ Links ]
16. Gottwein E, Cullen BR. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe 2008;3:375-387. doi: 10.1016/j.chom.2008.05.002. [ Links ]
17. Grivennikov SI, Greten F, Karin M. Immunity, inflammation,and cancer. Cell 2010;140:883-899. [ Links ]
18. Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 2009;27:693-733. doi: 10.1146/annurev.immunol.021908.132641. [ Links ]
19. Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol 2009;19:404-413. doi: 10.1016/j.tcb.2009.05.006. [ Links ]
20. Chaturvedi M, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kB addiction and its role in cancer: 'one size does not fit all'. Oncogene 2011;30:1615-1630. doi: 10.1038/onc.2010.566. [ Links ]
21. Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 2006;25:6831-6843. [ Links ]
22. Hoffmann A, Xia Y, Verma IM. Inflammatory tales of liver cancer. Cancer Cell 2007;11:99-101. [ Links ]
23. Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF, et al. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med 2008;205:2551-2560. doi: 10.1084/jem.20072581. [ Links ]
24. Ouellet DL, Provos P. Current knowledge of microRNAs and noncoding RNAs in virus-infected cells. Methods Mol Biol 2010;623:35-65. doi: 10.1007/978-1-60761-588-0_3. [ Links ]
25. Kim DN, Chae HS, Oh ST, Kang JH, Park CH, Park WS, et al. Expression of viral microRNAs in Epstein-Barr virus-associated gastric carcinoma. J Virol 2007;81:1033-1036. doi: 10.1128/JVI.02271-06. [ Links ]
26. Ghosh Z, Mallick B, Chakrabarti J. Cellular versus viral microRNAs in host-virus interaction. Nucleic Acids Res 2009;37:1035-1048. doi: 10.1093/nar/gkn1004. [ Links ]
27. Cullen BR. Viruses and microRNAs: RISCy interactions with serious consequences. Genes Dev 2011;25:1881-1894. doi: 10.1101/gad.17352611. [ Links ]
28. Moens U. Silencing viral microRNA as a novel antiviral therapy? J Biomed Biotech 2009. doi: 10.1155/2009/419539. [ Links ]
29. Edwards RH, Marquitz AR, Raab-Traub N. Epstein-Barr virus BART microRNAs are produced from a large intron prior to splicing. J Virol 2008;82:9094-9106. [ Links ]














