Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Boletín médico del Hospital Infantil de México
versión impresa ISSN 1665-1146
Bol. Med. Hosp. Infant. Mex. vol.67 no.5 México sep./oct. 2010
Artículo original
Impact of nutritional support on length of hospitalization and mortality in children after open heart surgery
Nalleli Vivanco-Muñoz,1 Alfonso Buendía-Hernández,2 Juan Osvaldo Talavera Piña,3 Antonio Juanico-Enríquez,4 Patricia Clark Peralta1
1 Clinical Epidemiology Unit, Hospital Infantil Federico Gómez; Faculty of Medicine, UNAM, México, D.F., México
2 Pediatric Cardiology, Instituto Nacional de Cardiología Ignacio Chávez, México, D.F., México
3 Clinical Epidemiology Research Unit, Hospital de Especialidades CMN SXXI, IMSS; Centro de Investigación en Ciencias Médicas, UAEMEX, México, D.F., México
4 Pediatric Intensive Care Unit, Instituto Nacional de Cardiología Ignacio Chávez, México D.F., México
Correspondence to:
M. Sc. Nalleli Vivanco-Muñoz
E-mail: nutricionpediatrica@hotmail.com
Received for publication: 06-01-2010.
Accepted for publication: 07-30-2010.
Abstract
Background. Malnutrition is a common cause of morbidity in children with congenital heart disease (CHD). The aim of this study was to assess the impact of malnutrition and nutritional support on the length of hospitalization and mortality at the Pediatric Intensive Care Unit (PICU) in children with CHD after undergoing surgery.
Methods. Clinical records (2000-2008) of patients £3 years old with CHD who were admitted for surgery were evaluated for nutritional status, nutritional support, and risk factors. Mortality was evaluated from the beginning of surgery and during the patient's stay at the PICU. Long-term hospitalization was considered according to the length of hospital stay on percentile >50. A multiple logistic regression model was used.
Results. Two hundred eighty nine patients were included. Factors related to mortality were malnutrition before surgery (OR 3.447; 95% CI 1.006–11.812, p = 0.049), early or delayed enteral nutrition (OR 0.007; 95% CI 0.000–0.097, p = 0.000, and OR 0.011; 95% CI 0.001–0.126, p = 0.000, respectively), and early parenteral nutrition (OR 0.032; 95% CI 0.002–0.452, p = 0.000) vs. no nutritional support. Factors related to long-term stay were malnutrition at birth (OR 2.772; 95% CI 1.282–5.995, p = 0.010) and delayed parenteral nutrition (OR 12.049; 95% CI 1.626–94.724, p = 0.015).
Conclusion. Malnutrition at birth and before surgery increases length of stay and mortality of children after open heart surgery. Early nutritional support reduces length of stay and mortality.
Key words: pediatric cardiology, surgery, nutrition, Pediatric Intensive Care Unit.
Introduction
Malnutrition is a common cause of morbidity in children with congenital heart disease (CHD).1-11 Despite advances in intensive cardiologic treatment, these children have a torpid evolution including prolonged mechanical ventilation, longer stay at the Pediatric Intensive Care Unit (PICU), increased rate of infections, reinterventions, reintubation, and early mortality (within 30 days).12-19
Although many techniques have been developed to rapidly correct the malformation, mortality remains at 10%.20-22 Recently, malnutrition has been found to be a strong risk factor for many complications such as infection, longer stay at the PICU, reintubation and death, among others.23 However, no studies have reported the prevalence and determinants of a long stay and mortality in the PICU related to malnutrition and nutritional support in our population. Such information is needed to design nutritional interventions for these children. Therefore, we designed this study to report the prevalence of these variables and the effect on length of stay and mortality at PICU.
Patients and methods
This is a retrospective cohort study. Clinical records of children with CHD <3 years old who underwent their first surgical corrective intervention with extracorporeal circulation from January 2003 to August 2008, at the Instituto Nacional de Cardiología "Ignacio Chávez" were reviewed. Forty nine percent of the charts were excluded for the following reasons: nonelective surgery, congenital syndrome, did not require extracorporeal circulation during surgery, had already had a previous major surgery, had been hospitalized for other causes, charts were incomplete, and had esophageal atresia. The total charts eligible were 490 and, of these, 289 were included.
A comprehensive evaluation of determinants for a long stay at the PICU and death was performed. These included information on demographics, birth history, cardiac status, socioeconomic class, and pre-, intra-, and postsurgical factors as well as nutritional support at the PICU. Malnutrition was defined as <90% of weight/age at birth using National Centre for Health Statistics (NCHS) charts and a Z-score of <−2 for body mass index (BMI) before surgery.24
Evaluated variables
According to the chronology of the event, the variables were grouped into four periods: 1) before hospitalization: sociodemographic (socioeconomic status) and clinical variables (sex and cardiologic diagnosis); 2) pre-surgical: anthropometric and medical treatment; 3) intrasurgical: time of aortic clamp, transfusions, bleeding, inotropics infused, and adverse events (bleeding, arrhythmia, cardiac arrest and cardiogenic shock); and 4) postsurgical: fasting, nutrition support infusion (early <72 h fasting, delayed >73hs fasting) enteral or parenteral nutrition, intubation, and transfusions. Long stay (>6 days) and death (before or after 72 h at the PICU) were evaluated as outcomes.
Statistical analysis
To test the association between nutritional status and treatment with long stay at the PICU and death, a bivariate analysis was done using χ2; all covariates were also evaluated. Later, two groups of independent multivariate analysis were conducted: 1) for evaluating nutritional status (early and late death patients were included), and 2) for evaluating nutritional treatment (only late mortality patients were included). Both analyses were adjusted for every variable collected. For nutritional diagnosis, the analysis was adjusted by baseline, pre-and intrasurgical variables (age, sex, socio-economic status, prematurity, Risk Adjustment for Congenital Heart Surgery (RACHS), presurgical transfusions, adverse events, bleeding, time of aortic clamp, inotropics). For nutritional support variables, analysis was done adjusting for the baseline, pre-, intra-, and postsurgical variables (age, sex, socioeconomic status, prematurity, RACHS, presurgical transfusions, adverse events, bleeding, time of aortic clamp, inotropics, infection, and prolonged mechanical ventilation (only for mortality).
In both analyses, each variable was contrasted against long stay and death at the PICU. In all cases, p £0.05 was considered significant (SPSS v.12 for Windows).
The study was approved by the Ethics and Research Committee Board of the institution.
Results
Four hundred ninety medical records of children submitted to corrective intervention with extracorporeal circulation for CHD were evaluated and 289 (59%) were included. The median age was 10.6 months (range: 0.1-36 months). There were 56% infants (≤ year old) and 44% were >1 year if age. The sample included 51.5% boys and 48% girls. We reported a long stay (>6 days) at the PICU in 50.5% of cases and a mortality rate of 15.5%.
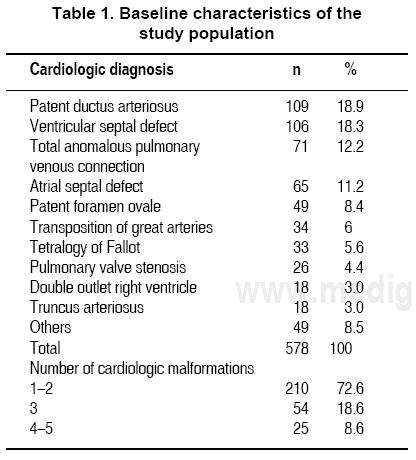
The most common cardiac diagnoses were patent ductus arteriosus, ventricular septal defect, and total anomalous pulmonary venous connections. The frequency of each malformation is listed in Table 1. Because many patients had more than one malformation, there was a total frequency of 578 diagnoses. Children were categorized into one of four groups according to cardiac diagnosis: a) acyanotic with increased pulmonary blood flow, b) acyanotic with normal pulmonary blood flow, c) cyanotic with increased pulmonary blood flow or d) cyanotic with diminished pulmonary blood flow. These diagnoses were made a priori by the attending physician using X-ray, echocardiography and in some cases hemodynamic procedures (Table 1).25
Bivariate analysis
The prevalence of long stays at the PICU was 25.6% of the acyanotic with increased pulmonary blood flow (AIPF) patients, 38.9% of the acyanotic with normal pulmonary blood flow (ANPF), 59.2% for the cyanotic with increased pulmonary blood flow (CIPF) group, and 76.3% of the cyanotic with decreased pulmonary blood flow (CDPF) group (p = 0.000). Mortality was reported in 5.8% of the AIPF group, 22.2% in the ANPF group, 17.6% in the CIPF group, and 26.3% in the CDPF group (p = 0.002) (Table 2).
The prevalence of prematurity in our study was 15%, of which 44.7% had a long stay and 10.5% died. Of the full-term children, 51.4% had a long stay and 16.2% died (p = 0.445 and 0.367).
Nutritional status at birth was evaluated: 46.5% of patients with normal weight had a long stay, whereas 56.9% of low-weight patients had a long stay (p = 0.112). There was a mortality rate of 13.4% in the normal-weight children and one of 19.6% of those with low weight (p = 0.151). Regarding nutritional diagnosis before surgery, 47.6% of the normal BMI children, 53.2% of the malnourished children, and 36.4% of overweight children had long stays (p = 0.345). Mortality was reported in 13.7% of the normal BMI children, 17.5% of the malnourished children, and 9.1% of the overweight children (p = 0.372) (Table 2).
Long stay according to the nutritional support received was as follows: for early and delayed enteral nutritional support, early and delayed parenteral support and no nutritional support: 11.4, 50, 69, 95.7 and 62.5%, respectively (p = 0.000). Mortality was reported in 1, 2.9, 6.9, 21.7 and 31.3% of each group, respectively (p = 0.000) (Table 2).
Multivariate analysis
In the multivariate analysis, malnutrition at birth increased the risk for long stay (OR 2.772; 95% CI 1.282–5.995; p <0.010), and malnutrition before surgery increased the risk for mortality (OR 3.447; 95% CI 1.006–11.812; p <0.049). They were adjusted for every variable involved in the outcome (Tables 3 and 4).
Nutritional therapy also showed an impact on both outcomes; delayed parenteral support increased the risk for a long stay in the PICU (OR 12.409; 95% CI 1.626–94.724, p = 0.015) and early enteral support had a protective effect on the same outcome (OR 0.11; 95% CI 0.026–0.472, p = 0.003) compared with patients without nutritional support (Tables 3 and 4). For mortality, early parenteral nutrition (OR 0.032, 95% CI 0.002–0.452, p = 0.011), delayed enteral support (OR 0.011; 95% CI 0.001–0.126, p = 0.000), and early enteral nutrition (OR 0.007; 95% CI 0.007–0.097, p = 0.000) were found to have a protective effect compared with those patients without any nutritional support, reaching statistical significance. They were also adjusted for every group of variables involved in the outcome (pre- and intrasurgical variables, nutritional status at birth and before surgery, age, sex, socioeconomic status, prematurity, RACHS, presurgical transfusions, adverse events, bleeding, time of aortic clamp, and inotropics) (Tables 3 and 4).
Discussion
This study shows a strong association between nutritional diagnosis and nutrition support for the surgically treated CHD pediatric patient with a long stay, as well as mortality at the PICU. Our results show the independent and statistical effects of malnutrition and nutritional support on both outcomes. Among the included variables, cardiologic diagnosis, RACHS, aortic clamp time, mechanical ventilation, and infection also appeared to be predictors for both outcomes (data not shown).
For a long stay at the PICU, early enteral nutrition acts as a protective factor. Malnutrition at birth and delayed parenteral support increased the risk for this outcome. Death was more prevalent among patients with malnutrition before surgery, and early enteral and parenteral nutrition had protective effects for that outcome. Sex of the patient and premature birth did not show any association with either outcome (data not shown).
Our study is different than those previously reported. Prognosis stratification was used for the logistic regression analysis. This method more precisely assesses the effect of malnutrition and nutrition support, considering every variable involved in the event, from birth to the medical treatment at the PICU.
For nutritional diagnosis before surgery, we chose the BMI Z score because, in our study, this proved to be a better predictive variable than weight, age, and height indexes developed by Waterloo and Gómez. This should be considered in new prognostic studies involving nutrition.26-31
It is important to consider that the effect of nutritional support on length of stay and mortality is closely related with the critical state of the patients. Parenteral support was the one of the best options for those patients with a dysfunctional gastrointestinal tract. This group and the group with no nutritional support included the sickest patients. To confirm this hypothesis, we performed a bivariate analysis in this group of patients showing that they had a RACHS score higher than the other groups, reflecting the worst prognosis (p = 0.000).
Our results agreed with those reported in the adult population where malnutrition was strongly associated with increased infection rates, late recovery, long mechanical ventilation support, and a long stay in the ICU.32-35 Marin et al. reported that some of the predictors for a long stay in the PICU are age <12 months, previous admissions, emergency admissions, and chronic treatment requirements (parenteral nutrition and tracheotomy), among others.33
With reference to nutritional support, several studies have reported that early parenteral nutrition in trauma patients increases infection and aggravates clinical outcomes. We found a similar association with long stay and delayed parenteral nutrition.34,36,37
Some of our results are similar to those reported in a recent study in Turkey, which associated eight risk factors for mortality at the ICU: age, APACHE II score (OR 1.99; 95% CI 1.5–2.64), mechanical ventilation (OR 1.98; 95% CI 1.33–2.95), length of stay in the ICU (OR 0.41; 95% CI 0.27–0.61), enteral nutrition (OR 0.43; 95% CI 0.29–0.65), tracheotomy (OR 0.26; 95% CI 0.094–0.75), steroid use or chemotherapy (OR 1.61; 95% CI 1.13–2.29), nosocomial pneumonia, and sepsis (OR 0.59; 95% CI 0.38–0.92).32
Additionally, regarding previous results we found that time of fasting prior to nutritional support is important. An increased risk for mortality in the delayed parenteral support group but not in the early group—was found in our patients. However, in the adult population, some metaanalysis and systematic reviews that did not find such association have concluded that parenteral nutrition support has no effect on mortality, only on the infection rate.38,39
Early enteral nutrition (before 24 h of admission) decreases the infection rate. Intrapyloric enteral nutrition as an alternative for those patients at risk for bronchoaspiration secondary to mechanical ventilation has been reported as a positive maneuver.40,41 On an experimental level, the advantages of enteral nutrition over parenteral nutrition are due to the use of substrates through the gastrointestinal route, which improves the local and systemic immune response and retains the barrier function of the bowel.42,43
Study limitations
The information was extracted from clinical charts; therefore, the measures and evaluations cannot be verified. However, clinical records show the usual clinical practice, and in certain way this reflects the effectiveness of the measure in real life. The clinical reasons for leaving a patient fasting or choosing parenteral support over enteral nutrition were not fully reported in the clinical charts. No clinical differences, however, were observed in patients with enteral or parenteral nutritional support.
The study also has several strengths including sample size, patient homogeneity, inclusion of almost any variable involved in the event chronologically, and the multivariate model that allowed us to discriminate between the effect of malnutrition and nutrition support on length of stay and mortality independent of the many variables affecting the outcome. Evidence-based information of the increased risk for a long stay or mortality due to malnutrition or nutrition therapy on these children may modify the clinician's treatment, improving the evolution and prognosis for these children.
As other publications, we were able to only report retrospective results. The need for prospective clinical trials is clear, but the difficulties of this design regarding these topics prevent the different groups in carrying out these studies.
We conclude that our findings emphasize the importance of early detection of malnutrition and the use of postsurgical early nutritional support, preferentially enteral nutrition. This may also impact treatment costs in these patients.
Acknowledgments
The authors acknowledge L.N. Carolina Angulo Vega.
References
1. Clark EB. Etiology of congenital cardiovascular malformations: epidemiology and genetics. In: Allen HD, Clark EB, Gutgesell HP, Driscoll DJ, eds. Moss and Adams' Heart Disease in Infants, Children and Adolescents, Including the Fetus and Young Adult. Philadelphia: Lippincott Williams & Wilkins, 2001. pp. 65-79. [ Links ]
2. Rosenthal GR. Incidence and prevalence of congenital heart disease. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. Baltimore: Williams & Wilkins, 1998. pp. 1083-1085. [ Links ]
3. Azevedo VM, Albanesi-Filho FM, Santos MA, Castier MB, Tura BR. The impact of malnutrition on idiopathic dilated cardiomyopathy in children. J Pediatr (Rio J) 2004;80:206-211. [ Links ]
4. Martins da Silva V, de Oliveira Lopes MV, Leite de Araujo T. Evaluation of the growth percentiles of children with congenital heart disease. Rev Lat Am Enfermagem 2007;15:298-303. [ Links ]
5. Mitchell IM, Logan RW, Pollock JC, Jamieson MP. Nutritional status of children with congenital heart disease. Br Heart J 1995;73:277-283. [ Links ]
6. Peterson RE, Wetzel GT. Growth failure in congenital heart disease: where are we now? Curr Opin Cardiol 2004;19:81-83. [ Links ]
7. Salzer HR, Haschke F, Wimmer M, Heil M, Schilling R. Growth and nutritional intake of infants with congenital heart disease. Pediatr Cardiol 1989;10:17-23. [ Links ]
8. Thompson COC, Reyes TN, Rabiela BOL, Buendía HA, Miranda CI, Carrasco QR. The nutritional status of the child with congenital cardiopathy. Arch Inst Cardiol Mex 1998;68:119-123. [ Links ]
9. Varan B, Tokel K, Yilmaz G. Malnutrition and growth failure in cyanotic and acyanotic congenital heart disease with and without pulmonary hypertension. Arch Dis Child 1999;81:49-52. [ Links ]
10. Villasís-Keever MA, Aquiles Pineda-Cruz R, Halley-Castillo E, Alva-Espinosa C. Frequency and risk factors associated with malnutrition in children with congenital cardiopathy. Salud Publica Mex 2001;43:313-323. [ Links ]
11. Mustafa I, Leverve XM. Metabolic and nutritional disorders in cardiac cachexia. Nutrition 2001;17:756-760. [ Links ]
12. Nichols DG, Ungerleisder RM, Spevak PJ, Greeley WJ, Cameron DE, Lappe DG, et al. Perioperative monitoring. In: Nichols DG, Cameron DE, eds. Critical Heart Disease in Infants and Children. Baltimore: Elsevier Health Sciences, 2006. pp. 479-506. [ Links ]
13. Engelman DT, Adams DH, Byrne JG, Aranki SF, Collins JJ Jr, Couper GS, et al. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg 1999;118:866-873. [ Links ]
14. Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition 1996;12:23-29. [ Links ]
15. Kulier A, Levin J, Moser R, Rumpold-Seitlinger G, Tudor IC, Snyder-Ramos SA, Moehnle P, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation 2007;116:471-479. [ Links ]
16. Potapov EV, Loebe M, Anker S, Stein J, Bondy S, Nasseri BA, et al. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur Heart J 2003;24:1933-1941. [ Links ]
17. Ray DE, Matchett SC, Baker K, Wasser T, Young MJ. The effect of body mass index on patient outcomes in a medical ICU. Chest 2005;127:2125-2131. [ Links ]
18. Davis S, Cox AC, Piedmonte M, Drummond-Webb JJ, Mee RB, Harrison AM. Prolonged mechanical ventilation after cardiac surgery in young children: incidence, etiology and risk factors. J Intensive Care Med 2002;17:302-307. [ Links ]
19. Yap CH, Mohajeri M, Yii M. Obesity and early complications after cardiac surgery. Med J Aust 2007;186:350-354. [ Links ]
20. Lechner E, Weisinger-Eidenberger G, Weissensteiner M, Hofer A, Tulzer G, Sames-Dolzer E, et al. Open-heart surgery in premature and low-birth-weight infants--a single-centre experience. Eur J Cardiothorac Surg 2009;36:986-991. [ Links ]
21. Dorfman AT, Marino BS, Wernovsky G, Tabbutt S, Ravishankar C, Godinez RI, et al. Critical heart disease in the neonate: presentation and outcome at a tertiary care center. Pediatr Crit Care Med 2008;9:193-202. [ Links ]
22. Pedersen KR, Hjortdal VE, Christensen S, Pedersen J, Hjortholm K, Larsen SH, et al. Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int Suppl 2008:S81-S86. [ Links ]
23. Pierro A, Eaton S. Metabolism and nutrition in the surgical neonate. Semin Pediatr Surg 2008;17:276-284. [ Links ]
24. Duggan C, Walker WA, Hendricks KM. The critically ill patients. In: Walker WA, ed. Manual of Pediatric Nutrition. Hamilton, Ontario: Decker; 2005. [ Links ]
25. Attie F, Buendía-Hernández A, Zabal C. Introducción. In: Attie F, Buendía-Hernández A, Zabal C, eds. Cardiología Pediátrica, Diagnóstico y Tratamiento. México: Médica Panamericana, 1994. pp. 26-31. [ Links ]
26. Centers for Disease Control (CDC). Pediatric Reference Growth Charts, 2000. Available at: http://www.cdc.gov/growthcharts/2000 [ Links ]
27. WHO. Child Growth Standards, 2007. Available at: http://www.who.int/childgrowth/standards/second_set/technical_report_2.pdf [ Links ]
28. Seal A, Kerac M. Operational implications of using 2006 World Health Organization growth standards in nutrition programmes: secondary data analysis. BMJ 2007;334:733. [ Links ]
29. Zeferino AM, Barros Filho AA, Bettiol H, Barbieri MA. Monitoring growth. J Pediatr (Rio J) 2003;79(suppl 1):S23-S32. [ Links ]
30. Eto C, Komiya S, Nakao T, Kikkawa K. Validity of the body mass index and fat mass index as an indicator of obesity in children aged 3-5 year. J Physiol Anthropol Appl Human Sci 2004;23:25-30. [ Links ]
31. Mackie AS, Gauvreau K, Newburger JW, Mayer JE, Erickson LC. Risk factors for readmission after neonatal cardiac surgery. Ann Thorac Surg 2004;78:1972-1978. [ Links ]
32. Colpan A, Akinci E, Erbay A, Balaban N, Bodur H. Evaluation of risk factors for mortality in intensive care units: a prospective study from a referral hospital in Turkey. Am J Infect Control 2005;33:42-47. [ Links ]
33. Marcin JP, Slonim AD, Pollack MM, Ruttimann UE. Long-stay patients in the pediatric intensive care unit. Crit Care Med 2001;29:652-657. [ Links ]
34. Chen YC, Lin SF, Liu CJ, Jiang DD, Yang PC, Chang SC. Risk factors for ICU mortality in critically ill patients. J Formos Med Assoc 2001;100:656-661. [ Links ]
35. Gilio AE, Stape A, Pereira CR, Cardoso MF, Silva CV, Troster EJ. Risk factors for nosocomial infections in a critically ill pediatric population: a 25-month prospective cohort study. Infect Control Hosp Epidemiol 2000;21:340-342. [ Links ]
36. Cagatay AA, Ozcan PE, Gulec L, Ince N, Tugrul S, Ozsut H, et al. Risk factors for mortality of nosocomial bacteraemia in intensive care units. Med Princ Pract 2007;16:187-192. [ Links ]
37. Heyland DK, McDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA 1998;280:2013-2019. [ Links ]
38. Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition 2004;20:843-848. [ Links ]
39. Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med 2005;31:12-23. [ Links ]
40. García Vila B, Grau T. Early enteral nutrition in the critically-ill patient. Nutr Hosp 2005;20:93-100. [ Links ]
41. Mesejo A, Juan M, García-Simón M. Enteral access and intestinal function assessment in the critically ill patient. Nutr Hosp 2007;22(suppl 2):37-49. [ Links ]
42. Radrizzani D, Bertolini G, Facchini R, Simini B, Bruzzone P, Zanforlin G, et al. Early enteral immunonutrition vs parenteral nutrition in critically ill patients without severe sepsis: a randomized clinical trial. Intensive Care Med 2006;32:1191-1198. [ Links ]
43. Sena MJ, Utter GH, Cuschieri J, Maier RV, Tompkins RG, Harbrecht BG, et al. Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. J Am Coll Surg 2008;207:459-467. [ Links ]














