Introduction
The worldwide prevalence of congenital heart disease is estimated at approximately nine out of 1000 live births, which has an important geographical variability. Although in developed countries, the prevalence of complex congenital heart diseases has decreased due to fetal screening, in developing countries, it is increasing1. In addition, cardiologists are increasingly attending to adult patients with congenital heart disease due to improved survival, treatments, and diagnostic tools.
Congenital anomalies of the pulmonary artery (PA) can range from an asymptomatic incidental finding to being the cause of sudden cardiac death, and this corresponds to the underlying vascular abnormality. In general, they are divided into (a) valvular/perivalvular anomalies, (b) abnormal narrowing, (c) abnormal origin/course, and (d) abnormal communications2,3. For an adequate study and approach to these pathologies, in addition to clinical suspicion, a multimodal imaging study is needed.
Clinical case
A 26-year-old male without the previous medical history arrived at the emergency room due to 3 months of progressive dyspnea with peripheral and central cyanosis. Physical examination showed normal blood pressure, rapid regular pulse (110 bpm), low blood oxygen of 75%, without clinical signs of respiratory insufficiency, cyanosis, and finger clubbing; precordial examination with findings suggestive of pulmonary hypertension. Electrocardiogram showed sinus rhythm associated with signs of the dilated right atrium and right ventricular hypertrophy due to systolic overload (Fig. 1A). Chest radiography showed an enlarged right atrium and enlarged pulmonary trunk (Fig. 1B).

Figure 2 Electrocardiogram and chest X-ray. ECG shows right axis deviation, giant P waves, and right ventricular hypertrophy with strain pattern (A). Chest X-ray demonstrated right atrium enlargement and left pulmonary flow increased with a prominent main and left pulmonary artery (B).
Laboratory findings showed significant erythrocytosis, thrombocytopenia, elevated NT-ProBNP, and D-dimer. Based on the initial findings, the differential diagnosis included Ebstein's anomaly, atrial septal defect, ventricular septal defect, pulmonary shunt, or stenosis in addition to ruling out pulmonary thromboembolism.
Transthoracic echocardiography showed a dilated right atrium and a dilated and hypertrophic right ventricle (free wall thickness 12.5 mm) with reduced systolic function (right ventricular fractional area change of 19%) and a high probability of pulmonary hypertension (estimated systolic pulmonary artery pressure of 161 mmHg) (Fig. 2A). Anomalous origin of the right branch of the pulmonary artery was also evidenced (Fig. 2B). A chest contrast-computed tomography (CT) with render volume 3D reconstruction was performed, in which patent ductus arteriosus (PDA) Krichenko Type E and anomaly origin of the right pulmonary artery (AORPA) from the ascending aorta were diagnosed (Fig. 3A-E). To establish a definitive diagnosis of pulmonary hypertension, we decide to perform cardiac catheterization (Fig. 2C and D) where we found a systolic pulmonary artery pressure of 136 mmHg, and a mean pulmonary artery pressure of 93 mmHg, which were higher than systemic pressure; the pulmonary vascular resistance (PVR) was 24.14 WU/m2, and right atrial pressure of 18 mmHg. A vasoreactivity test with inhaled nitric oxide was performed without significant change in pressure. The coronary arteries did not have any anatomical defects on coronary angiography. With these results, it was possible to establish the diagnosis of Eisenmenger syndrome, supplemental oxygen and sildenafil for pulmonary hypertension were started. The patient continued to follow-up in the congenital heart disease and pulmonary hypertension department.
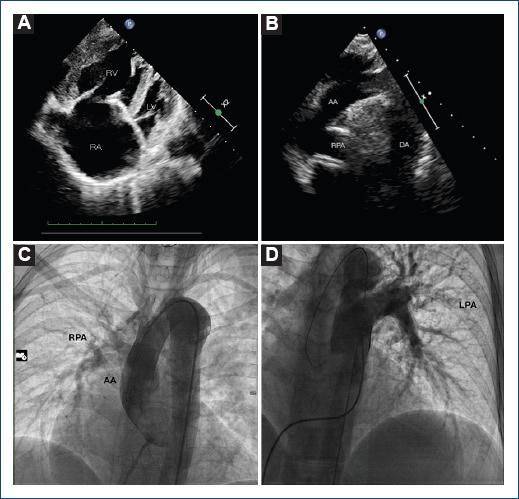
Figure 2 Transthoracic echocardiography and cardiac catheterization. Echocardiography showing apical 4-chamber view giant right atrium and hypertrophied right ventricle (A). The suprasternal view shows the right branch of the pulmonary artery arising directly from the ascending aorta. (B) Left heart catheterization and right branch of the pulmonary artery emerging from the aorta. (C) Right heart catheterization showing only the left branch arising from the trunk of the pulmonary artery (D).RA: right atrium; RV: right ventricle; LV: left ventricle; RPA: right pulmonary artery; AA: ascending aorta; LPA: left pulmonary artery.
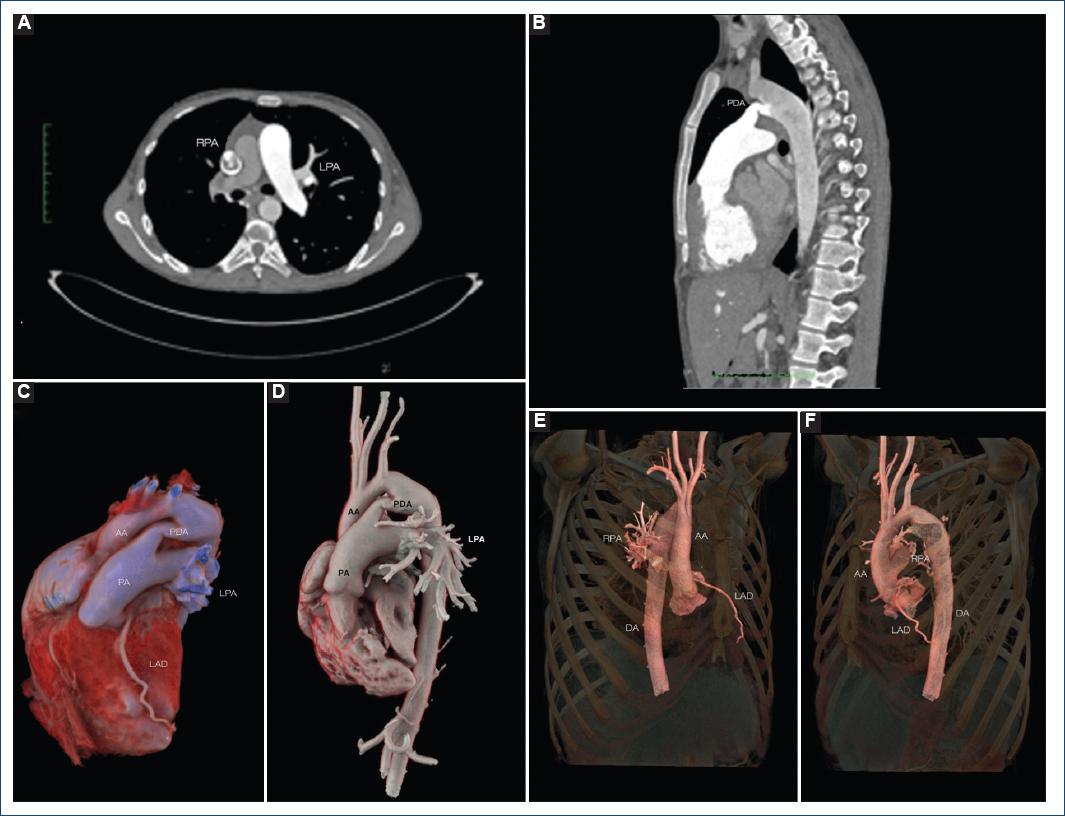
Figure 3 Computed angiotomography and 3D reconstruction. Computed axial tomography shows the abnormal origin of the right pulmonary artery from the aorta (A) and a patent ductus arteriosus (PDA) (B). Volume rendered images showing the PDA Type E Krichenko and the left branch of the pulmonary artery emerging from the trunk of the pulmonary artery (C and D) while the right branch of the pulmonary artery arises from the ascending aorta (E and F).PDA: patent ductur arteriosus; RPA: right pulmonary artery; LPA: left pulmonary artery; AA: ascending aorta.
Discussion
AORPA is a rare congenital cardiac anomaly characterized by the anomalous origin of one of the branch pulmonary arteries from the ascending aorta and a normal origin of the other PA from the right ventricular outflow tract in the presence of two semilunar valves4. The contralateral lung receives all the cardiac output in addition to the occasional blood flow of the associated anomalies, such as PDA, aortopulmonary window, interatrial, and interventricular septal defects, which may occur in approximately 40% of cases5.
The estimated incidence is about 0.1% among all congenital heart diseases with most AORPA cases diagnosed during infancy and only 5% reported in adults6. AORPA has a reported mortality rate as high as 70% among those patients who did not undergo surgical repair within one year of life; the main cause of the high mortality rate was pulmonary arterial hypertension (PAH) and irreversible pulmonary vascular disease7. Usually, chest radiograph findings are cardiomegaly and increased pulmonary vascular flow in the ipsilateral lung to AORPA; however, our patient had greater pulmonary flow in the contralateral lung due to the right-to-left shunt for advanced Eisenmenger syndrome.
Echocardiography is considered the first-line imaging tool to diagnose AORPA; nevertheless, CT angiography can provide important anatomic details and pre-operative planning3.
Heart catheterization is the gold standard for PAH and Eisenmenger syndrome. Acute vasoreactivity testing is particularly important in congenital heart disease with PAH, as it informs prognosis and can guide the correct treatment8.
In untreated patients with AORPA, the presence of the left to right shunt leads to a progressive increase in PVR causing pulmonary hypertension. With the progression of the disease, bidirectional shunting occurs, which turns into a predominant right-to-left shunt with further worsening of the disease. Thus, once the diagnosis is confirmed, surgery should be performed immediately, regardless of the patient's age, but irreversible pulmonary hypertension with Eisenmenger syndrome is a contraindication for surgery9.
Although recent improvements in the management of Eisenmenger syndrome, including the use of advanced therapies for PAH, have substantially increased life expectancy, long-term survival remains poor and is even lower in treatment-naive patients10.
Complex congenital heart disease reaching adulthood without repair is still prevalent in low-middle income countries. AORPA is a rare disease with a low survival rate without early surgical repair. A multimodality imaging approach with echocardiography and CT angiography is essential for diagnosis.











 nueva página del texto (beta)
nueva página del texto (beta)


