Introduction
Atrial flutter (AFL) is a macro-reentrant atrial tachyarrhythmia characterized by 240-400 beats/min atrial rates, an ECG pattern with P-waves replaced by saw-tooth type waves, and the absence of an isoelectric line; its risk increases with age and cardiovascular disease1,2. Even though the incidence of AFL is not precisely known, studies predict it to be present in 10% of patients with supraventricular tachycardia3,4. Electrical and structural remodeling, including interstitial atrial fibrosis and localized anomalies of the conduction system resulting from chronic arrhythmias, favored atrial enlargement and was documented in AFL5-7. In the long run, the chronicity of this disease could potentially lead to sinus node dysfunction8.
Sinus node (SN) dysfunction, a term that encompasses various rhythm anomalies and accounts for nearly 50% of pacemaker implantations in the United States, has been associated with atrial arrhythmias, including AFL9. Cardiovascular diseases such as ventricular hypertrophy also result in electrical and anatomical remodeling and can influence arrhythmias' development and progression10,11. These arrhythmias have been treated with many methods to prevent chronicity and further advance cardiac damage. Radiofrequency catheter ablation is a cornerstone in AFL treatment, with a long-term success rate of up to 95% of the cases12. There have been reported cases of atrial fibrillation (AF) where reverse remodeling occurs after a successful ablation, even in patients with previous SN dysfunction13. On the other hand, it is unclear if this phenomenon prevents future pacemaker requirements or if this data can be extrapolated to AFL.
In patients, the coexistence and chronicity of AFL and SN dysfunction can result in various clinical manifestations before deciding on a definitive diagnosis. Several tools have been used for diagnosing sinus node dysfunction, such as sinus node pause, sinus node recovery time (SNRT), and corrected sinus node recovery time (CSNRT), with limited data found regarding their use in AFL11,14. In addition, it is unclear whether detecting a prolonged CSNRT after a successful ablation could predict whether patients with subclinical SN dysfunction would require future pacemaker implantation14-16. Therefore, in this study, investigators would like to explore the prognostic value of CSNRT regarding the necessity for a pacemaker in patients with AFL who undergo catheter ablation.
Methods
Study design and population
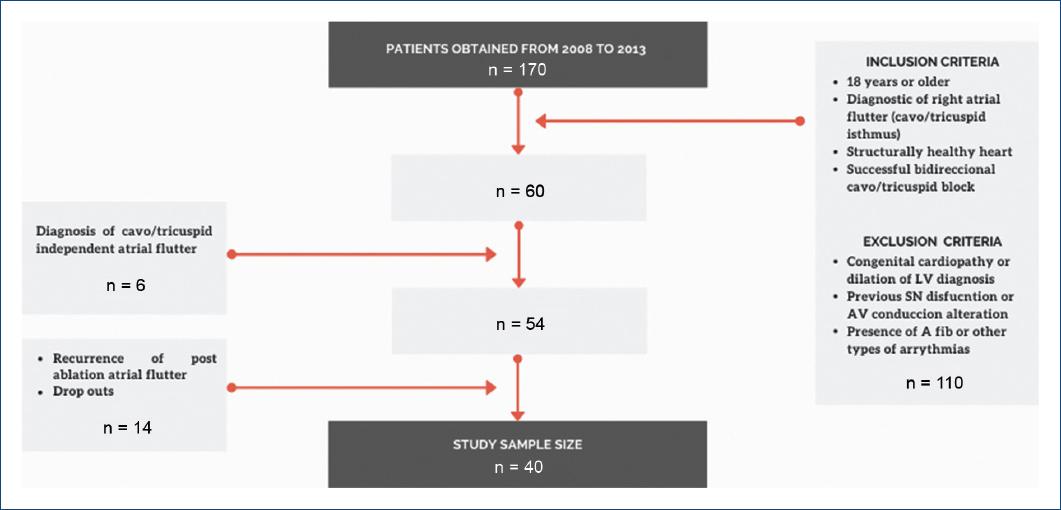
This prospective observational cohort study was conducted at the National Institute of Cardiology, "Ignacio Chavez" in Mexico City from 2008 to 2013 with a 5-year follow-up. The study size was determined by convenience; non-random sampling was used. Evaluation of the patient's physical and electronic files helped collect demographic data, clinical history, relevant past pathological history, data from the electrophysiological study, ablation procedure, and follow-up notes. Figure 1 shows the inclusion and exclusion criteria for this study group.
The dependent variable monitored was the requirement of a pacemaker implant. Strict admission criteria could limit the interpretation of the results to a small population with a precise presentation. Among the possible bias, we recognize the high prevalence of complex cases due to the study being in a 3rd level center. The lack of a proper previous database on these patients forced us to do a non-random convenience consecutive sampling due to the unknown incidence of cases.
Ablation procedure
A femoral puncture was performed with the placement of intravascular metal guides, which were replaced by two introducers. A decapolar electrode catheter was advanced through one of the introducers and was placed in the coronary sinus. Through the other introducer, a 20-pole electrocatheter was positioned around the tricuspid annulus. A tetrapolar electrode catheter was advanced through the catheter and placed at the apex of the right ventricle.
Stimulation was begun, decreasing by 16 beats until an arrhythmia was induced. Once the arrhythmia was triggered, the AFL activation sequence was verified. The electrode catheter was replaced by an ablation electrocatheter with external irrigation. Subsequently, an ablation line was placed between the tricuspid ring and the inferior vena cava until the cessation of the arrhythmia; then, the bidirectional blockade of the isthmus was confirmed. In the event of the persistence of the cavotricuspid isthmus conduction gap, the ablation line was continued until the disappearance of atrial potentials. All the ablation procedures performed found isthmus-dependent atrial flutter.
Assessment of intrinsic CSNRT
The autonomic nervous system influences heart rate dynamically, where the heart rate increases or decreases due to adrenergic tone; patient-external and internal factors such as anxiety, pain, and degree of relaxation can affect heart rate. An autonomic blockade was performed to neutralize these effects by administering atropine (0.03 mg/kg) and esmolol (500 mcg/kg) until a stable intrinsic sinus rate was evident. The procedure continued with stimulation of the SN using electrodes 5-6 of the 20-pole Halo electrocatheter with a pacing duration of 30-60 s followed by 1 min rest and a decreasing cycle length from 600 ms to 500 ms and finally 400 ms. In these sequences, SNRT was recorded, and later, the CSNRT was obtained by subtracting the basal sinus cycle length from the SNRT; any value > 550 ms in any sequence was considered abnormal. The introducers were removed, and compression was performed for 20 min, verifying the absence of hematomas or murmurs; the patient was transferred to the recovery room for 24 h. All antiarrhythmic medications were withdrawn from patients before the procedure.
Follow-up
The detection of AFL recurrence would lead to a second ablation, following the same procedure and consequent follow-up. Patient follow-up was conducted by medical consult a week after the process; posteriorly, the patient had regular appointments 3, 6, and 12 months later; afterward, annually for 5 years. At each visit, a surface EKG and a 24 h Holter were performed. During this period, we searched for subjective or objective data on the degenerative disease of the node. Patients who presented the following symptoms of suspicion for the development of SN dysfunction: syncope, reduced exertional capacity, inability to perform typical physical work, and dyspnea with physical effort were studied with an additional Holter or stress test. The requirement of a permanent pacemaker was defined as the detection of the following:
− Inability to increase heart rate in relation to the degree of effort in a stress test
− Exertional tolerance in stress test lower than expected for age
− Detection of sinus pause > 2.5 s in a 24 h Holter that correlates with symptoms
− Detection of episodes of atrial tachycardia-bradycardia in a 24 h Holter.
If no evidence of SN dysfunction arose during follow-up, an extra electrophysiological study was done. On confirmation of the diagnosis, the requirement of a pacemaker implant was confirmed, and a bicameral DDDR pacemaker was posteriorly implanted according to this study protocol.
Statistical analysis
Statistical analysis was executed using the SPSS V. 16.0 software. Continuous variables were presented as means and standard deviation (SD) when normal distribution was determined; otherwise, median and ranges were used. The qualitative variables were presented as absolute and relative frequencies. Qualitative variables and comparison of groups were analyzed using Student's T distribution for independent samples. Pearson's χ2 test or Fisher's test was used for categorical variables.
Results
Initially, 170 patients were eligible for this study, but the sample was 40 patients due to inclusion and exclusion criteria, dropouts, cavotricuspid independent atrial flutter, and recurrence of post-ablation atrial flutter (Fig. 1). These were subdivided into two study groups during data analysis depending on their requirement of pacemaker implant post-SN ablation. All general demographic data are shown in table 1, where 24 participants were male (60%). Fourteen participants (35%) out of the sample size required a pacemaker implant (P group), these having an average age of 50.7 ± 16.7 years in comparison to 55.9 ± 14.2 years in the no pacemaker group (NP group). The mean heart rate in the NP group was 78.26 ± 17.26 versus 92.14 ± 40.08 in the P group. The left ventricle ejection fraction (LVEF) did not differ significantly across both groups, with a value of 54.21 ± 10.38 versus 58.5 ± 10.3 % for p = 0.13, since severe degree of heart failure was an exclusion criteria for this study.
Table 1 Patient characteristics and the need for pacemaker implant
| Variables | Pacemaker implant | ||
|---|---|---|---|
| Yes (n = 14) n (%) | No (n = 26) n (%) | p-value | |
| Age (years) | 50.71 ± 16.7 | 55.96 ± 14.2 | 0.45 |
| Sex | |||
| Male | 7 (50%) | 17 (65.38%) | 0.34 |
| Female | 7 (50%) | 9 (34.61%) | |
| Comorbidities | |||
| Diabetes | 2 (14%) | 2 (7.69%) | 0.5 |
| Hypertension | 3 (21%) | 9 (34.6%) | 0.38 |
| Symptoms | |||
| Palpitation | 10 (71.4%) | 22 (84.62%) | 0.455 |
| Lipothymia | 3 (21.4%) | 2 (7.69%) | |
| Dyspnea | 1 (7.14%) | 2 (7.69%) | |
| Treatment | |||
| Beta-blockers | 3 (21.4%) | 12 (46.15%) | 0.12 |
| Antiarrhythmic | 7 (50%) | 13 (50%) | 1.0 |
| SND DX time (years) | 10.42 ± 8.18 | 10.03 ± 11 | 0.45 |
| DX method | |||
| ECG | 8 (57.1%) | 18 (69.23%) | 0.62 |
| Holter | 5 (35.7%) | 5 (19.23%) | |
| Electrophysiology study | 1 (7.14%) | 2 (7.69%) | |
| Stress test | 0 | 1 (3.85%) | |
SND DX: sinus node dysfunction diagnosis time; ECG: electrocardiogram.
The comorbidities found in this study included 4 patients (10%) who suffered from diabetes mellitus; 50% required a pacemaker implant. Twelve patients (30%) had a history of hypertension; 25% required a pacemaker implant. Regarding the flutter type diagnosed in each patient, 87.5% had a counterclockwise arrhythmia versus the 12.5% clockwise flutter; 85.71% of the participants who required a pacemaker had a counterclockwise flutter diagnosis.
CSNRT > 550 ms is considered the prognostic variable for pacemaker implants in patients diagnosed with AFL who undergo an ablation. In our study, the NP group had a mean CSNRT of 383.54 ms ± 67.96 ms, while the P group obtained 1972.57 ms ± 3423.56 ms (p ≤ 0.01). Ten patients (25%) had a CSNRT > 550 ms, out of which 100% required a pacemaker; thus, 10 out of the 14 patients (71.43%) who required pacemaker implants had an elevated CSNRT, resulting in a statistically significant variable (p ≤ 0.01).
Measurements of SN function variables are depicted in table 2. SN dysfunction was diagnosed in 13 participants (32.5%), 10 of which (71.43%) required a pacemaker, while 4 participants (28.57%) with a normal SN function also required pacemakers (p ≤ 0.01). The post-ablation SN pause was statistically significant (p = 0.0069): the P group had a mean SN pause duration of 1.86s ± 0.96 s in comparison to the NP group, 1.196 s ± 0.52 s. Sinus pause > 2.5 s is a diagnostic parameter of SN dysfunction; however, most of our patients did not present this finding.
Table 2 ECG findings and the need for pacemaker implant
| Variables | Yes (n = 14) n (%) | No (n = 26) n (%) | p-value |
|---|---|---|---|
| ECG HR (bpm) | 92.14 ± 40.08 | 78.26 ± 17.2 | 0.97 |
| ECG QRS | 90.71 ± 18.59 | 95.38 ± 25.3 | 0.829 |
| ECG rhythm | |||
| Sinus | 7 (50%) | 11 (42.31%) | 0.64 |
| Flutter | 7 (50%) | 15 (57.69%) | |
| ECG alterations | |||
| None | 10 (71.43%) | 15 (57.69%) | 0.49 |
| LBBB | 2 (14.29%) | 2 (7.69%) | |
| RBBB | 2 (14.29%) | 7 (26.92%) | |
| RBBB+anterior | 0 | 2 (7.69%) | |
| fascicle | |||
| Flutter type | |||
| Counterclockwise | 12 (85.71%) | 23 (88.46%) | 0.8 |
| Clockwise | 2 (14.29%) | 3 (11.54%) |
ECG: electrocardiogram; HR: heart rate; Bpm: beats per minute; RBBB: right bundle branch block; LBBB: left bundle branch block.
CSNRT > 550ms is considered the prognostic variable for pacemaker implants in patients diagnosed with AFL who undergo an ablation. In our study, the NP group had a mean CSNRT of 383.54ms ± 67.96ms, while the P group obtained 1972.57ms ± 3423.56ms (p = < 0.01) (Table 3). Ten patients (25%) had a CSNRT > 550ms, out of which 100% required a pacemaker; thus, 10 out of the 14 patients (71.43%) who required pacemaker implants had an elevated CSNRT, resulting in a statistically significant variable (p = < 0.01).
Table 3 Procedural findings and need for a pacemaker implant
| Variables | Yes (n = 14) n (%) | No (n = 26) n (%) | p-value |
|---|---|---|---|
| LVEF | 54.21 ± 10.38 | 58.5 ± 10.3 | 0.13 |
| CSNRT | 1972.57 ± 3423.55 | 383.5 ± 67.95 | < 0.01* |
| Post-ablation SN pause | 1.86 ± 0.96 | 1.19 ± 0.52 | 0.006* |
| SN function | |||
| Normal | 4 (28.57%) | 23 (88.47%) | < 0.01* |
| Dysfunction | 10 (71.43%) | 3 (11.54%) | |
| ECG HR > 100 bpm | |||
| Yes | 4 (28.57%) | 2 (7.69%) | 0.078 |
| No | 10 (71.43%) | 24 (92.31%) | |
| Sinus pause > 2.5 s | |||
| Yes | 3 (21.43%) | 1 (3.85%) | 0.077 |
| No | 11 (78.57%) | 25 (96.15%) | |
| CSNRT > 550 ms | |||
| Yes | 10 (71.43%) | 0 | < 0.01* |
| No | 4 (28.57%) | 26 (100%) | |
| PR > 200 ms | |||
| Yes | 1 (7.14%) | 6 (23.08%) | 0.206 |
| No | 13 (92.86%) | 20 (76.92%) | |
| Flutter recurrence | 2 (14.29%) | 8 (30.77%) | 0.251 |
BMP: beats per minute; s: seconds; ms: milliseconds; CSNRT: corrected sinus node recovery time; HR: heart rate; SN: sinus node; FEV1: forced expiratory volume.
Discussion
AFL has gained attention in medical studies over the past two decades due to many factors, including its importance as a prevalent supraventricular arrhythmia, increasing studies on electrical and structural remodeling caused by arrhythmias, and the development of radiofrequency ablation as a definitive treatment. Despite this, the evidence for the association between atrial arrhythmias and SN dysfunction is derived mainly from atrial fibrillation (AF) studies. It has not been consistently studied in medical literature whether SN dysfunction persists after successful ablation and posterior pacemaker implantation in patients with AFL despite the evidence of reverse atrial remodeling after successful ablation and sinus rhythm maintenance17,18.
Our study was composed of 24 male participants (60%) out of 40 and was divided into two groups: the pacemaker group (P group) and the non-pacemaker group (NP). The average age in the P group was 50.7 ± 16.7 years compared to 55.9 ± 14.2 years in the NP group. Although age predisposes to the development of SN dysfunction and the need for a pacemaker, our study population's demographic did not support this hypothesis (p = 0.4520)19,20. The reason for this may be the dispersion, and relative heterogeneity in the ages of the patients who required pacemakers since 10 of them were under 60 years of age, and three of them were between 18 and 27 years of age. It is infrequent for patients in this age group to present with SN dysfunction; therefore, many differential diagnoses were evaluated and discarded before proceeding with the study.
The comorbidities associated with the development of SN dysfunction include a history of an atrial septal defect, coronary artery bypass graft surgery, low body mass index, longer flutter cycle length, and heart failure21. The comorbidities present in our study included diabetes and hypertension, which are involved in the genesis of arrhythmias in other studies, but our investigation did not focus on that aspect. Diabetes mellitus (DM) has been extensively studied, and many have produced inconsistent findings regarding its association with arrhythmias. The Framingham heart study, which conducted a 38-year follow-up of the participants, found that diabetic patients have a 40% greater risk of developing atrial fibrillation. This risk may be due to prolonged fluctuations in glucose levels causing autonomic alterations and cardiac remodeling22. By inducing myocardial changes, hypertension also has similar effects on the heart. The European Heart Rhythm Association (EHRA) and the European Society of Cardiology (ESC) Council on Hypertension published a review discussing the relationship between the two diseases23.
Ten patients in the P group had a CSNRT > 550 ms (1972.57 ms ± 3423.56 ms), and during follow-up, they were found to have SN dysfunction, leading to the requirement for pacemaker implantation. Researchers Chang et al. examined 34 patients who underwent catheter ablation to treat AF. The participants were divided into groups based on their need for pacemaker implantation post-ablation (Group 1 and Group 2). Patients in Group 1 had a greater incidence of pacemaker implantation than patients in Group 2. When the electrophysiological characteristics of each group were reviewed, it was revealed that the mean CSNRT value in Group 1 was 1042 ± 390 ms compared to 348 ± 125 in Group 2. Similar results were also observed in our study24. Other studies have shown a relationship between CSNRT and AF recurrence, considering it prognostic18. An investigation by Chen showed that 22 out of 159 patients with paroxysmal AF who underwent a radiofrequency catheter ablation procedure had AF recurrence; patients with a CSNRT > 550 ms had a higher recurrence rate than those with low CSNRT25. On the same line, Sairaku studied the relationship between flutter cycle length and CSNRT in patients with AFL. The authors reported that those who required pacemakers had a flutter cycle length of 295 + 37 ms and a CSNRT of 1727 + 1014 ms. Therefore, they concluded that flutter cycle length and CSNRT predicted SN dysfunction and pacemaker requirement. Our study group reported a shorter flutter cycle length (258.4 ± 64.7 ms) and a longer CSNRT (1972.5 ± 3423.5 ms), but the flutter cycle length did not achieve statistical significance, whereas CSNRT did. Our study is similar to Sairaku's findings in that both values are related to advanced degrees of atrial remodeling, and a prolonged CSNRT is expected in this population due to SN dysfunction26.
Among the strengths of this study is the use of strict inclusion criteria that increase internal validity and establish a reliable relationship between the variable CSNRT and the need of pacemaker implant in patients post-ablation. Similarly, using an objective variable to speculate a clinical outcome could be used clinically to prevent AFL recurrence. This study was conducted with a long time follow-up to acknowledge the short-, medium-, and long-term effects. On the other hand, the weaknesses include a restricted capacity to recognize confounding variables and their roles in our study; also, because the study was observational, inference of causality was limited.
Conclusion
In the present study, the authors have demonstrated that CSNRT is a prognostic tool that may be used to assess potential pacemaker implants in patients with sinus node dysfunction following ablation for AFL. AFL patients are commonly underdiagnosed for extended periods; once the diagnosis is reached, there is significantly advanced sinus node function alteration. Thus, efficient assessment tools like CSNRT that help diagnose these patients give physicians a chance to stop the progression of the disease; CSNRT gives clinicians means that offer better prognostic data for patients who suffer from this disease and provide timely treatment. It is recommended that CSNRT be evaluated as a predictive tool for the future need for pacemakers in patients with AFL.
Statement of ethics
This study was developed following the Declaration of Helsinki and the Belmont Report regarding respect toward patient rights and maintaining ethical standards from the planning to the publication of this scientific work. The Institutional Educational Council served as the reviewing body and the supervisors of ethical and scientific standards. Informed consent was obtained from all individual participants included in the study.











 nueva página del texto (beta)
nueva página del texto (beta)