Introduction
According to the results reported in a cohort study carried out in a tertiary hospital in Mexico City, the mortality reported in patients who required admission to the intensive care unit was 49.2%, all of whom required mechanical ventilatory assistance; 45% of the patients who did not survive did not receive invasive mechanical ventilation (IMV) due to lack of availability1. Faced with a public health emergency that generates a demand for resources that cannot be satisfied, it is necessary to provide criteria to guide "triage" decision-making, which is a very complex scenario for health personnel2. The main obstacle to solving the lack of ventilators is their high costs and the limited availability in the market. To generate national innovative technological resources that allow us to face current epidemiological adversities, the conceptual, computational design, and manufacture of a prototype of portable basic ventilatory support was developed by automating an AMBU, as an emergency AID.
Materials and methods
The conceptual design began in March 2020, in the area of technological innovation of the INC and was advised by the engineering faculties of the UNAM and UAM to manufacture an automated ventilation device based on AMBU (ESSI-1 INC) in a rapid manner, at a low cost, with affordable materials.
The components of the ESSI-1 INC are listed in table 1. Its control system can moderate the AMBU compression frequency, at 15, 18, 21, 24, 27, and 30 breaths per minute (bpm).
Table 1 Components of the ESSI-1 INC
| 1 | Bag-type hand resuscitator (AMBU). |
| 2 | Positive pressure regulating valve at the end of expiration (PEEP). |
| 3 | Pressure relief regulating valve. |
| 4 | Ventilatory circuit. |
| 5 | High efficiency HEPA filter. |
| 6 | Compressor arms made by 3D printing (PLA/photosensitive resin) that compress the resuscitator (AMBU). |
| 7 | Linear actuator (electric piston motor). |
| 8 | R3 microcontroller card. |
| 9 | Ventilatory rate controller switch. |
| 10 | On/off switch. |
| 11 | Acrylic and aluminum box. |
| 12 | Rechargeable battery (lasting up to 5 h). |
The AMBU used for this protocol is a Hudson RCI brand; model 5374, however, the ESSI-1 INC can couple different brands. The AMBU has a connection for an oxygen intake, an adjustable PEEP valve, and a pressure relief safety valve for up to 60 cm H2O. A HEPA filter was also integrated into the system.
The tests performed on the device were as follows:
- Using the compensating spirometer (TISSOT) (Cardiopulmonary Physiology Department of the INC): we evaluated the tidal volume in three manual ventilation devices of different brands, for adults with similar volumes mounted on the ESSI-1 INC equipment.
- In the ventilator/artificial lung simulator/tester (model 5600i Dual Adult PNEU VIEW SYSTEM) from the biomedical department of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ): the programmed respiratory rate, the behavior of the tidal volume, pressure, volume, and flow curves, simulating physiological lung compliance and higher pressures were evaluated.
- Evaluation in an "ex vivo" model: two dissected anatomical complexes of pig "lung-trachea-bronchi" were obtained. The 8F endotracheal tube was introduced, sealing it by inflating the balloon to the trachea, the ESSI-1 INC was connected and we evaluated the mechanical behavior, the safety of all its components and connections, and thus subjectively evaluate the ventilatory dynamics (Fig. 1).
-
- Evaluation of the performance and oxygenation contribution through sensors of anesthesia equipment (AEONMED 7500 brand) measuring:
The inspired fraction of oxygen (FiO2) provided by the ESSI-1 INC according to the supply of the oxygen intake at different volumes supplied.
The Inspiration/Expiration (I/E) ratio according to the respiratory rate programmed automatically by the ESSI-1 INC.
The behavior of the tidal volume with variations in the programmed ventilation frequency.
The behavior of the pressure-volume-time curves compared with the anesthesia ventilation equipment.
- Tests in porcine animal models "in vivo": in the experimentation room of the INC. Two male Yorkshire pigs weighing 85 kg and 80 kg, respectively, were used. Under monitoring and surveillance by the veterinary team and the anesthesiology team, peripheral venous access was placed, peripheral arterial access was cannulated for monitoring invasive pressure, and orotracheal intubation was carried out, with a No. 8 tube. The pig was connected to the AEONMED 7500 anesthetic ventilatory support equipment and subsequently to the ESSI-1 INC, both programmed at 21 rpm with a FiO2 contribution of 5 lts/min, to compare the ventilatory parameters, and baseline hemodynamics, blood gas blood pressure, pulse oximetry, invasive blood pressure, and heart rate every 5 min. The position of the endotracheal tube was corroborated by fluoroscopy. In the first porcine model, we evaluated the mechanical behavior of the ESSI-1 INC, the maintenance and behavior of the volumes, and the blood gas parameters.
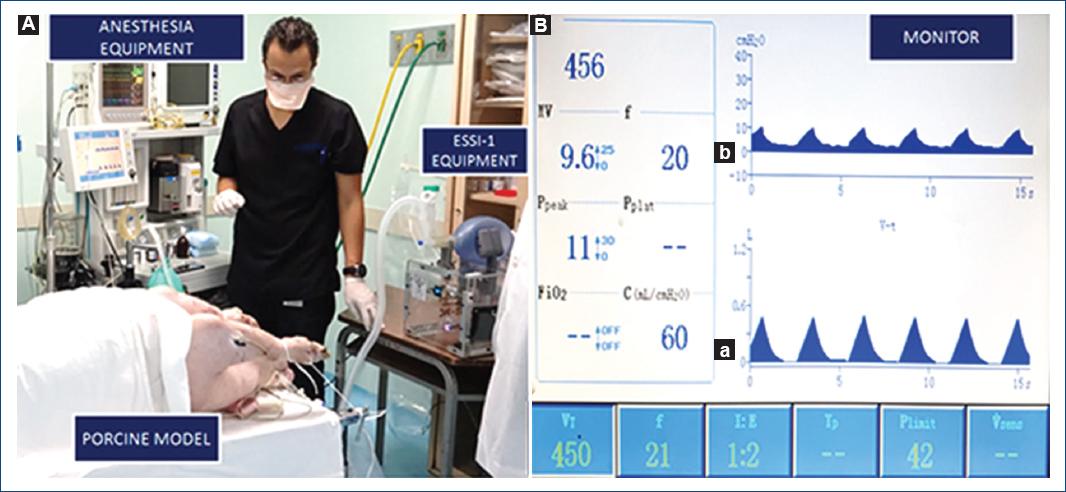
Figure 1 A: evaluation of ESSI-1 operation on "in vivo" porcine model and anesthesia equipment. B: characteristic time-dependent respiratory and current drain profiles of ESSI-1 connected to the anesthesia equipment (AEONMED 7500 brand): (a) pressure, (b) volume of the ESSI-1 device. Advanced volume support ventilation with VTID at 456 ml, I:E ratio 1:2, 21 BPM.
In the second, we also evaluated the behavior of the pressure, volume, and time curves, the behavior of the PEEP valve, and the overpressure regulation valve. After extubating, both pigs showed complete recovery from anesthesia and ventilatory assistance without complications.
The total duration of the experiment was 2 h with each one, with continuous work of the ESSI-1 INC device.
Type of connections:
In the first pig, we used a double tube system, in which the PEEP valve was located near the AMBU. Based on the blood gas results and guided by the bibliographic recommendations in the second pig, it was decided to change the configuration of the connections (prototypes of fans of an open design, shared by the Massachusetts Institute of Technology), eliminating the dead space of the system of expiration, and placing the PEEP valve very close to the endotracheal cannula3. This new configuration of the connections and elements of tubes, filters, and valves proved to be more efficient, with a substantial decrease in the dead space on expiration observed in the figure 2.
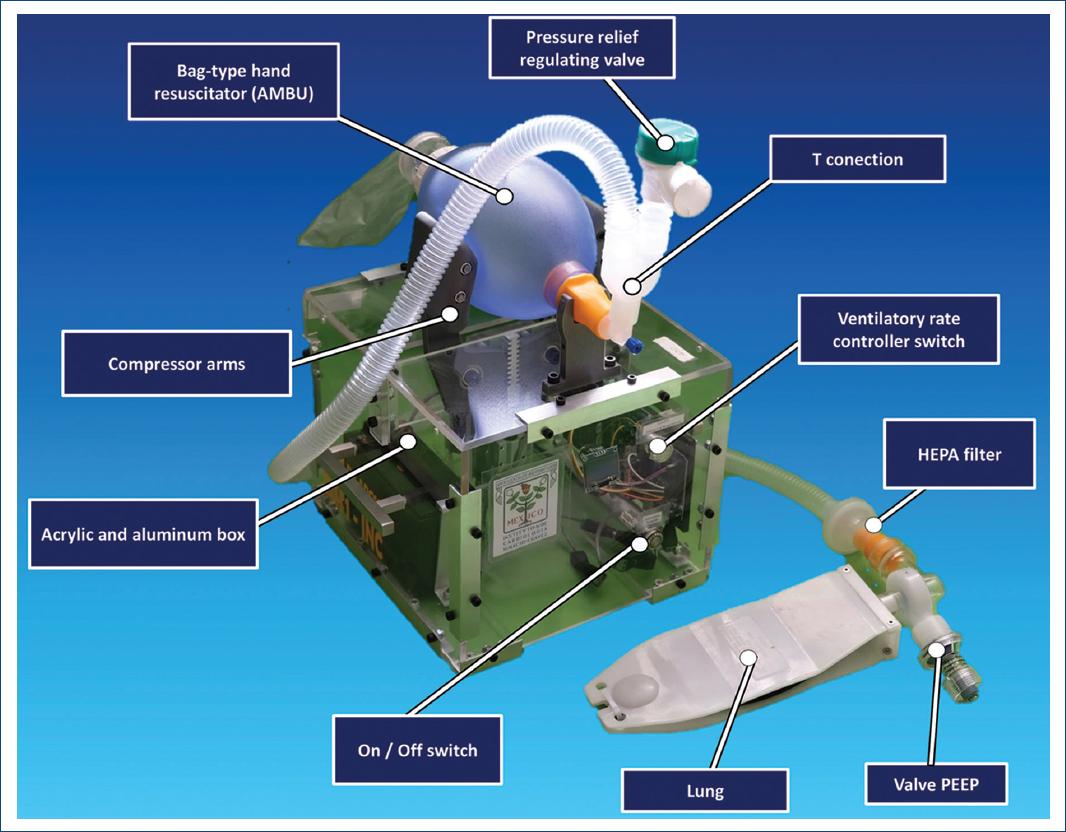
Figure 2 Visualization of functional ESSI 1, with connected valves. This shows an acrylic and aluminum body containing all electronic parts. The system has security connections, such as the overpressure safety valve and PEEP valve.
- A pilot test was carried out to evaluate the variability of the device: by measuring the parameters of ventilatory rate, tidal volume, and I/E relation for 10 min performed on a training manikin for airway management (laerdal brand) with average compliance of 30 cm H20, intubated with an 8F cannula and using the sensors of the AEONMED 7500 brand anesthesia machine with which variability4 of tidal volume values, pressure/time curves and respiratory rate of ventilation management using the same AMBU was compared manually by two medical interns versus management by the ESSI-1 INC (in which they set the ventilatory frequency parameters of 15/min to maintain tidal volume in the range of 360-420 mL and an I/E ratio of 1/2)5. Interns were instructed to try and maintain the same parameters.
Results
-
- Tidal volume test by TISSOT method:
The tidal volume was corroborated showing a minimal change in the tidal volumes produced by the equipment: 410 mL (Handi Brand), 420 mL (Hudson RCI Brand), and 439 ml (HEMC Brand).
- Ventilator simulator/tester/artificial lung: the respiratory rate was similar to that carried out by the ESSI-1 INC from 15 to 30 ventilations/min (Table 2). The pressure-volume-time curves were very adequate. The tidal volume averaged 430 mL and remained stable despite varying lung compliance from normal to high. Average intrapulmonary pressure of 10 cm H2O.
- Test by evaluating the anatomical model of the trachea complex, pig lung bronchi "ex vivo:" the behavior of the device from a mechanical and electrical point of view was satisfactory (maintenance of ventilatory frequency, mechanical behavior of the system, and autonomy due to battery for more than 12 h). The subjective evaluation of the distension of both lung models was considered satisfactory.
-
- Evaluation of the performance and oxygenation contribution by sensors of anesthesia equipment to the ESSI-1 INC. The behavior of the FiO2 provided by the ventilator according to the oxygen intake was consistent and adequate (Table 3).
The I/E ratio according to the programmed respiratory rate on average with the different respiratory rates evaluated was 1/2 with a ventilatory rate of 30 to 1/3 with a ventilation frequency of 21x.
The different pressure, volume, and time curves were similar between the programmed anesthesia equipment and the ESSI 1 INC.
-
- Tests in animal models:
Table 2 Results of the tests with the artificial lung of the Instituto Nacional de Ciencias Médicas y Nutrición "Salvador Zubirán" (INCMNSZ)
| Respiratory rate of the ESSI-1 INC | Min | 15 | 18 | 21 | 24 | 27 | 30 |
|---|---|---|---|---|---|---|---|
| Tidal volume | ML | 426 | 422 | 432 | 430 | 431 | 414 |
| Volume per minute | L | 6.33 | 7.62 | 8.96 | 10.11 | 11.33 | 11.99 |
| Inspiratory flow average | L/min | 15.3 | 17.4 | 17.8 | 17.7 | 17.4 | 15.2 |
| Inspiratory flow peak | L/min | 48.3 | 62.9 | 63.1 | 63.8 | 62.3 | 45.8 |
| Inspiratory time | S | 0.820 | 0.710 | 0.710 | 0.710 | 0.720 | 0.800 |
| Inspiratory radius | Range | 1:3.91 | 1:3.67 | 1:3.07 | 1:2.59 | 1:2.16 | 1:1.58 |
| Spontaneous respiratory rate | Min | 14.88 | 18.07 | 20.76 | 23.52 | 26.31 | 28.98 |
Table 3 FiO2 behavior according to the supply of O2 to the AMBU reservoir
| D Tank | ||
|---|---|---|
| L/min | FiO2 (%) | Min-duration |
| 0 | 21 | 101.01 |
| 5 | 55 | 73.60 |
| 7.5 | 73 | 49.07 |
| 10 | 92 | 36.80 |
| 15 | 98 | 24.53 |
The average heart rate in the control was 95/min and, with the ESSI-1 INC, it was 95. The peripheral saturation in the control averaged 92% and, with the ESSI-1 INC, it ranged from 85% to 90%. The control tidal volume was 322 mL and with the ESSI-1 INC 349 ml. The fluoroscopic image of the lungs showed a similar image on inspiration and expiration with both devices.
The blood gases are shown in table 4, which shows normal baseline control values and with a drop in oxygenation and respiratory acidosis in the three samples with the ESSI-1 INC equipment, not being able to reverse it despite adjustments in frequency and basic configuration of the connections. We concluded that the operation of the mechanical and electrical device was very satisfactory and reliable, the tidal volumes and respiratory rates were consistent as expected and that CO2 retention was due to dead space secondary to the type of connections. The total duration of the experiment was 2 h with continuous work of the ESSI-1 INC device. Changes were made to the disposition of the tubing to modify or eliminate expiratory dead space.
With the new configuration of more efficient connections and elements, tubes, filters, and valves, and the substantial reduction of dead space on expiration.
The average heart rate in the control was 95/min and with the ESSI-1 INC, it was 95. The peripheral O2 saturation in the control was an average of 95% and with the ESSI-1 INC, it remained at 95%, with a feeding of the reservoir bag of the AMBU at an O2 flow of 5 L/min. We found no differences in the behavior of blood gases in the basal state and with the ESSI-1 INC equipment, with a programmed respiratory rate of 21 rpm, and with a PEEP pressure of 5 cm H2O, both of which were normal results.
We observed a slight decrease in tidal volume, according to the modification of PEEP, obtaining the following results:
■ Basal PEEP (5 cm H2O)−Tidal volume: 595 mL.
■ PEEP 1 (10 cm H2O)−Tidal volume: 519 mL.
■ PEEP 2 (15 cm H2O)−Tidal volume: 440 mL.
Similarly, the modification in the tidal volume with the closing or opening of the overpressure relief valve was evaluated, with which it was observed:
■ Pressure of 60 cm H2O−Tidal volume: 615 mL.
■ Partial closure of the overpressure valve−Tidal volume: 461 mL.
■ Total closure of the overpressure valve−Tidal volume: 277 mL.
Comparative blood gas behavior of both pigs is observed in table 4.
- Pilot test to evaluate the variability of the "AMBU" device for manually assisted ventilatory assistance versus assisted through the ESSI-1 INC:
Table 4 Comparative table of blood gas behavior of pig # 1 and # 2 in the anesthesia machine and the ESSI-1 INC both at 10 min
| Models in-vivo | pH | pCO2 mmHg | pO2 mmHg | PaO2/FiO2 | HCO3 | Lactate |
|---|---|---|---|---|---|---|
| Pig #1 | ||||||
| Anesthesia machine | 7.38 | 50 | 305 | 300 | 28.9 | 1.7 |
| ESSI-1 INC | 7.17 | 89 | 178 | 178 | 33.1 | 1.2 |
| Pig #2 | ||||||
| Anesthesia machine | 7.4 | 46 | 346 | 346 | 1.1 | |
| ESSI-1 INC | 7.42 | 41 | 308 | 308 | 0.9 |
In pig # 1, CO2 retention and respiratory acidosis is observed, which is normalized in pig # 2 by modifying the connection system.
Ventilation performed with the ESSI-1 INC showed less variability compared to that performed manually by interns with AMBU ventilation training (Table 5). This is a pilot study of one in which we will evaluate this "variability" with a larger number of health personnel participants.
Table 5 Pilot test comparing the performance of the AMBU by automated management using the ESSI 1-INC versus manual compression by a medical intern at INC for 10 min
| Parameters | INC device | Medical intern |
|---|---|---|
| Mean tidal volume (mL) | 420.30 mL | 396.38 ml |
| L/min | 6.346 L/min | 4.687 L/min |
| Variability coefficient (%) of the tidal volume | 1.1 | 7.3 |
| Average ventilatory rate | 15.1 | 11.8 |
| I:E ratio | 1:1.84 | 1:2.44 |
Discussion
A percentage of seriously ill patients with mechanical ventilation criteria have been left without care due to its lack of availability and the mortality registered in Mexico City up to the moment. In regards to the patients who required mechanical ventilation, the percentage was 73.7%1. On the other hand, the time elapsed from ICU admission to intubation averaged 8 h and up to 25% were intubated 24 h after admission6 and the average number of days that required ventilatory assistance was 21.67.
It is important to develop devices that provide ventilatory assistance to the patient who require it as a temporary solution until a conventional mechanical ventilator is available, with better handling and safety. To this end, multiple attempts have been made to develop safe and effective devices8-11. The development of the ESSI-1 INC ventilatory assist device and its functional evaluation showed constant performance in maintaining satisfactory ventilatory volumes, evaluated using different methodologies already mentioned, which were maintained despite variations in ventilatory rate and lung compliance. The performance of the FiO2 contribution was able to increase from 21% without supplemental oxygen, up to 98% with 15 L/min.
In regards to safety, it was corroborated by arterial gas homeostasis with blood gases in pigs (Table 4) and the absence of barotrauma evaluated by fluoroscopy.
In addition to its evaluation in experimental biological models where the results demonstrated adequate mechanical and functional performance, it complied with the requirements to be considered an efficient and safe device according to what has been published by Columbia University, NYC12.
An important limitation of manual ventilatory assistance through AMBU is the variability registered in the performance of the operators (Table 5), which entails an increase in the adverse effects of ventilation, since excessive pressures and volumes can lead to barotrauma, volutrauma, gastric insufflation, and bronchoaspiration and when they are insufficient they condition hypoxia and hypoperfusion5; in our pilot study, the operators were asked to reach a rhythm of 15 ventilations/min, obtaining a mean of 11.8 (Table 5). This justifies developing devices capable of maintaining constant ventilatory parameters through AMBU and thus reducing complications.
Conclusions
The ESSI-1 INC device is a versatile, flexible, and innovative device that simulates ventilatory parameters of ventilatory rate, tidal volume, and I/E 1/2 ratio provided by a mechanical ventilator, at a significantly lower cost.
This type of low-cost device is justified only if there are no more complete and robust ventilators available, especially for the most serious patients due to COVID-1913, where precise adjustments of volumes, pressures, moisture, and PEEP are required. This development at the Instituto Nacional de Cardiología may be the beginning of alternative medical device projects in Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)


