Introduction
Atherosclerotic disease frequently affects the lower extremities arteries, affecting 10% in US adults older than 55 years old1. The symptoms related to atherosclerotic narrowing of the aorta or lower extremity arteries depend on the location and severity of disease, as muscle function is impaired. Indeed, reduced muscle perfusion, with subsequent impaired mitochondrial activity, increased muscle fat infiltration and fibrosis are pathogenic mechanisms related to worse symptoms2,3.
In addition to difference in size, histological differences arise between suprapatellar and infrapatellar arteries. Proximal arteries have a predominance of elastic component in the media, and progressively, muscular fibers predominate in more distal arteries media. Moreover, differences in shear stress according to the topography may impair differences in endothelial function4.
The previous studies have found contradictive results regarding the location of the arterial narrowing or occlusion, as proximal (aortoiliac) PAD compared with those with more distal PAD had a poorer general prognosis, independent of risk factors and comorbidities5, nevertheless, patients with diabetes or with end-stage kidney disease generally present with more distal disease, which are known risk factors of worse outcomes6,7. These clinical characteristics may be responsible for a worse clinical prognosis, behaving like confounders.
On the other hand, revascularization of infrapatellar stenosis may be more challenging due to a more diffuse, calcified disease, requiring generally more revascularization events compared to suprapatellar stenosis8-10.
Methods
Study design
This was a retrospective, observational cohort study designed and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement11.
Settings
The study was undertaken at the Hospital Italiano de Buenos Aires, which is a university hospital in Buenos Aires, Argentina. We retrospectively queried a database of adult patients who required revascularization due to symptomatic PAD from January 2014 to June 2020. The follow-up was considered from the first revascularization event to last medical contact or death of the patients.
Participants
Patients aged over 18 years at our institution and diagnosed with symptomatic PAD with further revascularization (either surgical or percutaneous) were included in the analysis. Iatrogenic, inflammatory or traumatic causes of revascularization events were excluded from the analysis, as atherosclerotic disease was the main focus of the analysis.
Variables
Symptomatic PAD was defined as a > 70% narrowing of the arteries of the limbs that produced pain and/or tissue loss (according to the clinical presentation). The stenoses locations were secondarily grouped into 3 anatomical levels: aorto-iliac arteries, femoral-popliteal arteries, and infrapatellar arteries. Furthermore, both aortoiliac and femoropopliteal stenosis were considered suprapatellar lesions. Infrapatellar stenosis was considered below the knee stenosis. Each patient could have 1 or more levels affected, with coexisting lesions in a same leg or in the other leg. Those patients with infrapatellar disease (irrespective of the presence of suprapatellar disease) were considered in the infrapatellar group. No distinction was made regarding the laterality of the lesion.
The risk factors, comorbidities, and treatments at the time of the angiography were collected from the medical charts, with baseline variables defined as follows: Diabetes was defined by a fasting blood glucose > 7 mmol/L at admission or the use of any oral antidiabetic agent and/or insulin. Hyperlipidemia was defined according to the documented patient's history and/or a fasting blood cholesterol > 240 mg/dL at admission. Patients were considered hypertensive if they took any antihypertensive drug. and/or if their average systolic blood pressure exceeded 140 mmHg or diastolic blood pressure exceeded 90 mmHg. The previous myocardial infarction (MI) was defined as any coronary ischemic event reported in the medical chart with requirement of coronary revascularization. The previous HF event was defined according to the documented medical history of admissions due to volume overload with requirements of intravenous diuretics.
Clinically, patients may present with intermittent claudication (IC), defined as a reproducible discomfort of the muscles of the legs induced by exercise and relieved with rest. Based on the timing of the inciting event, patients with threatened limbs are classified as acute ischemia (AI) (defined as symptoms that started in < 2 weeks) or chronic limb-threatening ischemia (CLTI) (defined as those symptoms that persisted > 2 weeks)12.
Outcomes
The primary outcome of interest was to compare the composite of hospitalizations due to chronic limb-threatening ischemia (CLTI) and major amputation events (defined as supramalleolar amputations) between infrapatellar and suprapatellar PAD patients12. CTLI hospitalizations were defined as hospital admissions due to the presence of PAD in combination with rest pain, gangrene, or lower limb ulceration beyond > 2 weeks of duration13. Secondary outcomes included the individual components of the primary endpoint, death, minor amputation events (i.e., distal to the forefoot), spontaneous MI events (defined by the fourth MI universal definition)14, and stroke (either ischemic - defined as brain ischemia due to thrombosis, embolism, or systemic hypoperfusion - or hemorrhagic due to intracerebral hemorrhage or subarachnoid hemorrhage)15. Major adverse cardiovascular events (MACE) were defined as the composite of death, MI, and stroke.
Statistics
Data were tested for deviation from Gaussian distribution using the Kolmogorov–Smirnov test. Normally distributed continuous variables are expressed as mean ± standard deviation, non-normally distributed continuous variables by median and interquartile range (IQR) in parentheses. Discrete variables are presented as number and percentages in parentheses. Categorical variables were compared using the Chi-square (χ2) test or Fisher exact test when appropriate. Continuous variables were compared with t-test or Mann Whitney U-test, as appropriate. Incidence risk ratios (IRR) are provided for all clinical endpoints in the analysis. Those variables that a priori were thought to be clinically relevant were included a univariable and in a multivariable logistic regression model. Cumulative incidence regarding the primary outcome was expressed by a Kaplan-Meier curve and compared using the log-rank test. Hazards ratios were estimated by means of Cox regression analysis. p < 0.05 was considered statistically significant. All analyses were performed by R v3.5.2 (R foundation for computational sciences, Vienna, Austria).
Ethical considerations
The study protocol was approved by our local internal review board. Patient informed consent was waived due to the retrospective nature of our study. Only members of the clinical team had access to routinely collected data, which were anonymized at the point of analysis. Our study is compliant with the principles outlined in the Declaration of Helsinki16.
Results
Patient characteristics
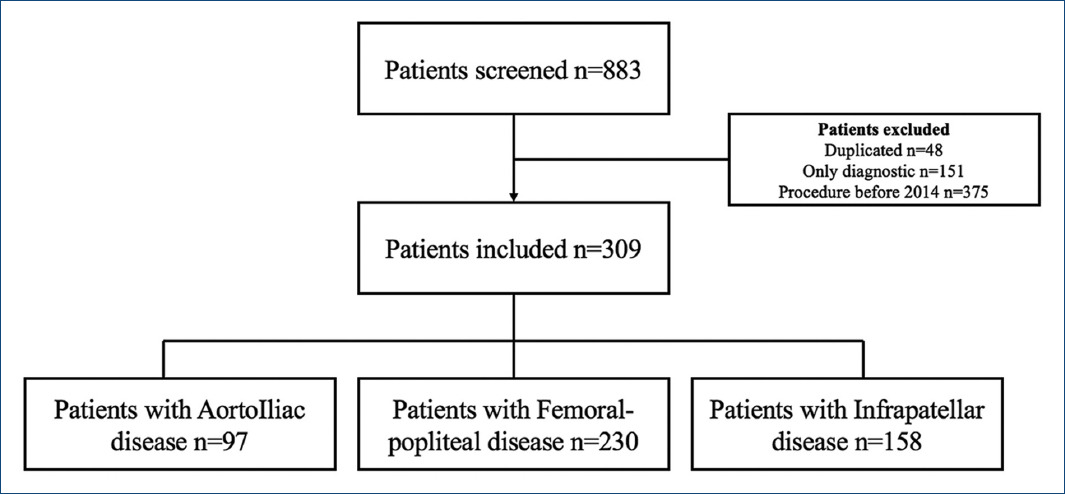
From January 2014 through July 2020, a total of 309 patients were included in the analysis after a thorough chart review of patients who met inclusion and exclusion criteria (Fig. 1). The median follow up was 1.87 years IQR (0.72-3.67 years). The mean age in our overall cohort was 71.84 ± 11.17 years old. About 64.7% were male and 30.5% were diabetic. IC was the most frequent clinical presentation (56%), followed by CLTI (29.4%) and AI (14.2%). Surgical revascularization was pursued in only 16.9% of the patients.
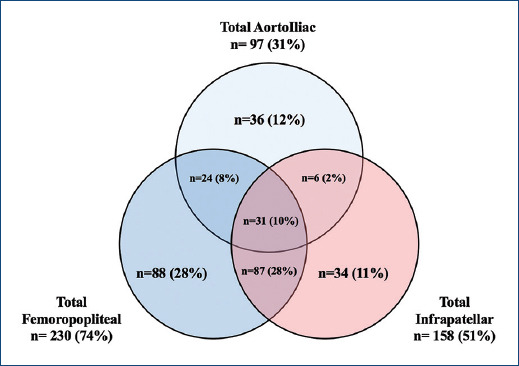
We further classified the patients regarding the location of the PAD. 97 (31.3%) patients had aortoiliac disease, 230 (74.4%) femoropopliteal, and 158 (51.1%) infrapatellar disease. These disease topographies frequently coexisted (Fig. 2). The baseline characteristics of the patients are found in table 1. Of our interest, 151 patients had suprapatellar disease, and 158 had infrapatellar disease. Infrapatellar disease patients had more prevalence of diabetes mellitus (38.2% vs. 22.5%, p < 0.05) and lower prevalence of tobacco consumption (13.9% vs. 30.5%, p < 0.05). Moreover, they were more likely to present with CLTI (38.6% vs. 19.9%, p < 0.05) (and thus higher Rutherford classification) and less likely to present with AI events (8.9% vs. 19.9%, p < 0.05).
Table 1 Demographic difference in suprapatellar and infrapatellar groups
| Variables | Overall (n = 309) | Suprapatellar (n = 151) | Infrapatellar (n = 158) | p-value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age mean ± SD | 71.84 ± 11.17 | 70.89 ± 11.30 | 72.76 ± 11.00 | 0.141 |
| BMI mean ± SD | 27.40 ± 5.07 | 26.99 ± 5.03 | 27.80 ± 5.09 | 0.171 |
| Male (%) | 200 (64.7) | 97 (64.2) | 103 (65.2) | 0.955 |
| Hypertension (%) | 257 (83.2) | 123 (81.5) | 134 (84.8) | 0.525 |
| Diabetes mellitus (%) | 94 (30.5) | 34 (22.5) | 60 (38.2) | 0.004 |
| Dyslipidemia (%) | 202 (65.8) | 103 (68.2) | 99 (63.5) | 0.449 |
| Active tobacco consumption (%) | 68 (22.0) | 46 (30.5) | 22 (13.9) | 0.001 |
| Chronic renal Injury (%) | 60 (19.6) | 28 (18.8) | 32 (20.4) | 0.837 |
| Prev. myocardial infarction (%) | 68 (22.2) | 32 (21.6) | 36 (22.8) | 0.915 |
| Prev. heart failure (%) | 34 (11.0) | 13 (8.6) | 21 (13.4) | 0.249 |
| Stroke (%) | 29 (9.4) | 14 (9.3) | 15 (9.6) | 1 |
| Atrial fibrillation (%) | 52 (16.9) | 19 (12.7) | 33 (20.9) | 0.076 |
| Laboratory | ||||
| Hematocrit mean ± SD | 37.21 ± 5.59 | 37.62 ± 5.41 | 36.81 ± 5.75 | 0.204 |
| Creatinine median (IQR) | 0.97 (0.76-1.2) | 0.96 (0.75-1.18) | 1 (0.78-1.30) | 0.339 |
| Clinical presentation | ||||
| Intermittent claudication (%) | 173 (56.0) | 90 (59.6) | 83 (52.5) | 0.256 |
| CLTI (%) | 91 (29.4) | 30 (19.9) | 61 (38.6) | 0.001 |
| Acute ischemia (%) | 44 (14.2) | 30 (19.9) | 14 (8.9) | 0.009 |
| Rutherford #4 (%) | 74 (23.9) | 44 (29.1) | 30 (19.0) | 0.050 |
| Rutherford #5 (%) | 58 (18.8) | 11 (7.3) | 47 (29.7) | 0.001 |
| Rutherford #6 (%) | 7 (2.3) | 2 (1.3) | 5 (3.2) | 0.481 |
| Surgical revascularization (%) | 52 (16.9) | 31 (20.7) | 21 (13.4) | 0.121 |
| Severe LVEF (%) | 9 (3.1) | 2 (1.4) | 7 (4.6) | 0.2 |
CLTI: chronic limb threatening ischemia; IQR: interquartile range; LVEF: left ventricle ejection fraction.
Clinical endpoints
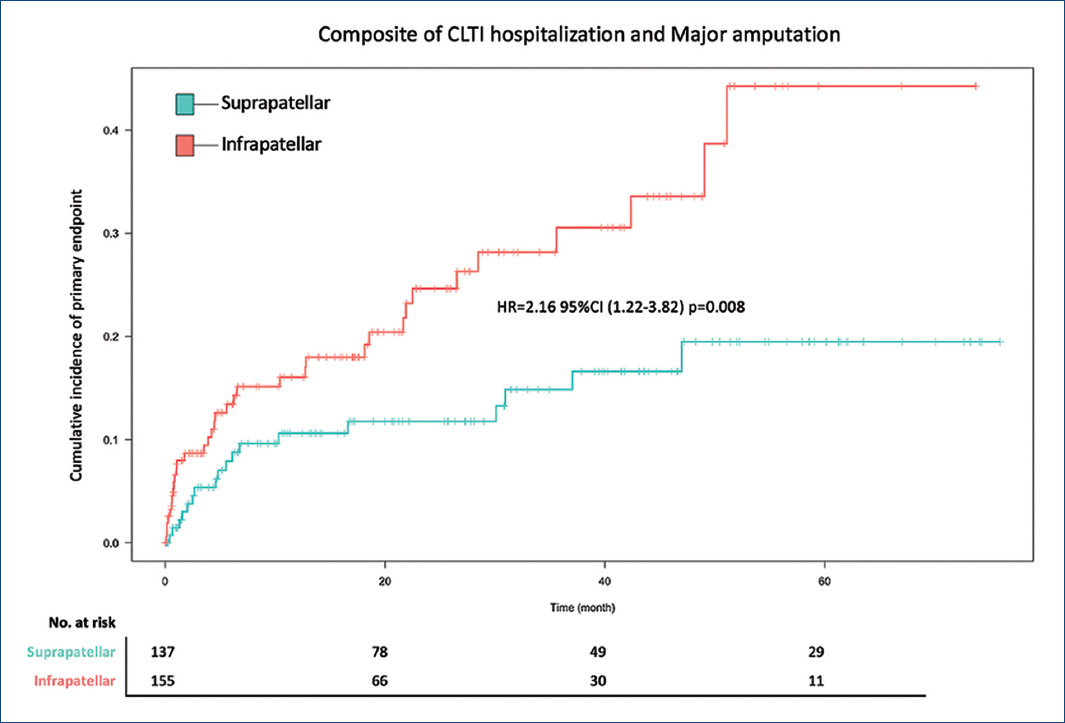
The primary composite of hospitalization of CLTI events and major amputation occurred in 35 patients (22.2%) in the infrapatellar patients and 18 patients (11.9%) in the suprapatellar patients (HR = 2.16 95% confidence interval [CI] = 1.22-3.82; p = 0.008) (Table 2 and Fig. 3).
Table 2 Clinical endpoints
| Variables | Suprapatellar (n = 151) | Infrapatellar (n = 158) | HR (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Patients with events (%) | IRR (per 100 person year) | Patients with events (%) | IRR (per 100 person year) | |||
| Primary outcome | ||||||
| Composite of hospitalization of CLTI and major amputations (%) | 18 (11.9) | 6.4 | 35 (22.2) | 11.8 | 2.16 (1.22-3.82) | 0.008 |
| Secondary outcomes | ||||||
| Hospitalization of CLTI (%) | 14 (9.3) | 4.9 | 26 (16.5) | 8.8 | 2.16 (1.12-4.15) | 0.021 |
| Major amputation (%) | 5 (3.3) | 1.8 | 16 (10.1) | 5.4 | 3.15 (1.15-8.6) | 0.025 |
| Death (%) | 23 (15.2) | 8.1 | 25 (15.8) | 8.5 | 1.3 (0.73-2.29) | 0.445 |
| Non-Fatal MI (%) | 7 (4.6) | 2.5 | 7 (4.4) | 2.4 | 1.31 (0.44-3.95) | 0.627 |
| Non-Fatal Stroke (%) | 4 (2.6) | 1.4 | 5 (3.2) | 1.7 | 1.43 (0.38-5.37) | 0.595 |
| MACE (%) | 30 (19.9) | 10.6 | 36 (22.8) | 12.2 | 1.43 (0.87-2.35) | 0.154 |
| Minor amputation (%) | 3 (2.0) | 1.1 | 15 (9.5) | 5.1 | 5.09 (1.47-17.6) | 0.010 |
95% CI: 95% confidence interval; CLTI: chronic limb threatening ischemia; HR: Hazard ratio; IRR: Incidence risk ratio; MACE: major adverse cardiovascular outcomes; MI: myocardial infarction.
Event rates for both components of the primary outcomes were increased in the infrapatellar patients. Of the infrapatellar patients, 26 (16.5%) were hospitalized due to CLTI, as compared with 14 (9.3%) in the suprapatellar group (HR = 2.16; 95% CI = 1.12-4.15; p = 0.021). Major amputation events occurred in 16 patients (10.1%) with infrapatellar disease and in 5 patients (3.3%) with suprapatellar disease (HR = 3.15; 95% CI = 1.15-8.6; p = 0.025) (Fig. 4).
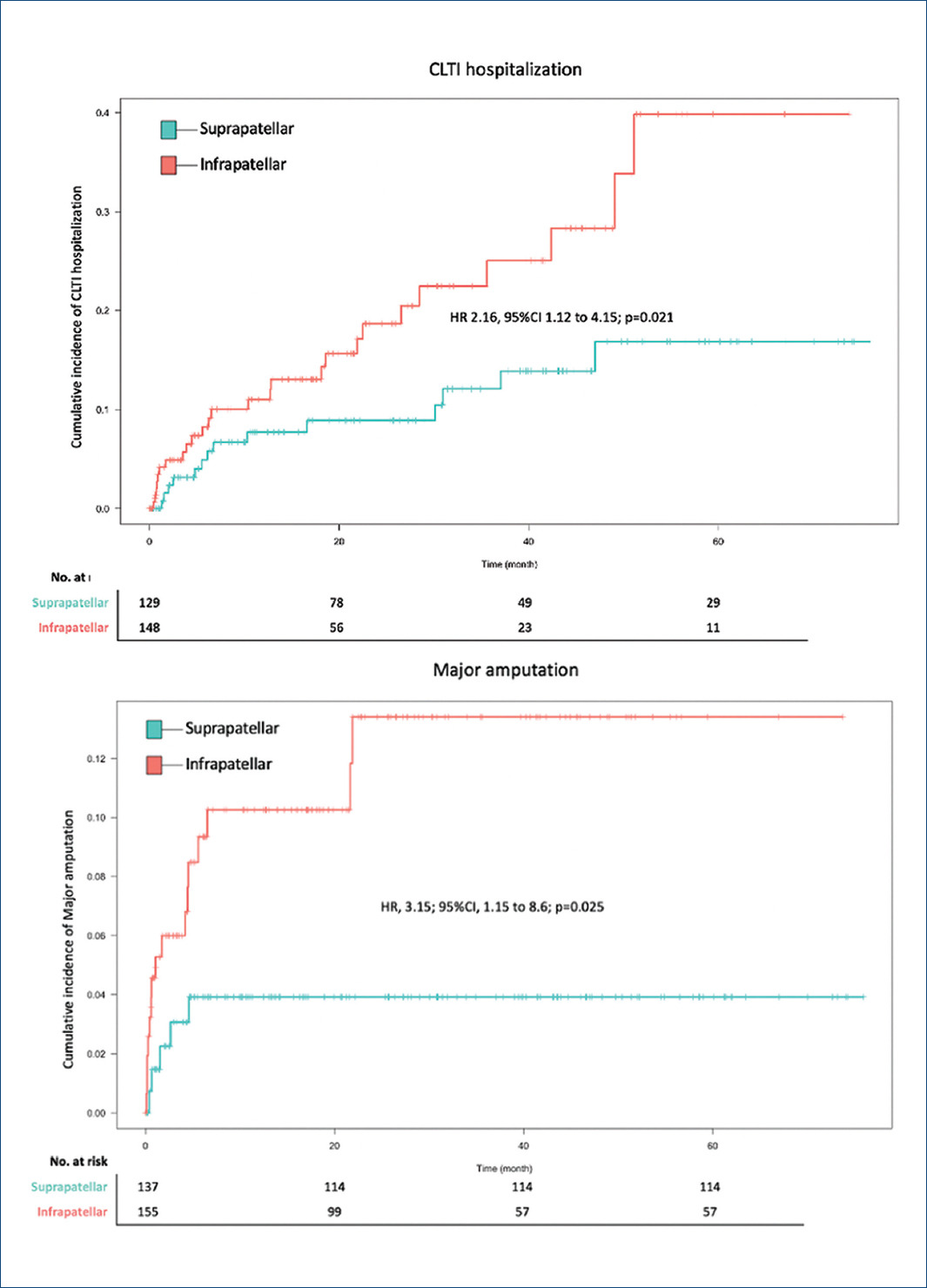
Figure 4 Cumulative incidence of both chronic limb threatening ischemia hospitalizations and major amputation events.
Death from all causes occurred in 25 patients (15.8%) with infrapatellar disease and in 23 patients (15.2%) with suprapatellar disease (HR = 1.3; 95% CI = 0.73-2.29; p = 0.445). Non-fatal MI occurred in 7 patients (4.4%) in the infrapatellar group and 7 patients (4.6%) (HR = 1.31; 95% CI = 0.44-3.95; p = 0.627). Non-fatal stroke occurred in 5 patients (3.2%) in the infrapatellar group as compared to 4 patients (2.6%) in the suprapatellar group (HR = 1.43; 95% CI = 0.38-5.37; p = 0.595). MACE events occurred in 36 patients (22.8%) in the infrapatellar group as compared to 30 patients (19.9%) in the suprapatellar group (HR = 1.43; 95% CI = 0.87-2.35; p = 0.154).
Minor amputation events occurred more frequently in the infrapatellar group (15 [9.5%]) than in the suprapatellar group (3 [2%]) (HR = 5.09; 95% CI = 1.47-17.6; p = 0.010).
After adjusting for diabetes, tobacco consumption, age, male sex, CLTI and AI, hematocrit, creatinine serum levels at presentation and surgical revascularization, infrapatellar PAD was independently associated with an increased risk for the primary composite endpoint (OR = 2.22; 95% CI = [1.05-4.81]; p = 0.038) (Table 3). We further performed a sensitivity multivariate regression model in which those with pure suprapatellar PAD was included, to compare pure infrapatellar and suprapatellar disease, finding still a worse prognosis in those with infrapatellar disease (OR = 2.33; 95% CI = [1-5.79]; p = 0.030) (Table 4).
Table 3 Univariate and multivariate, regarding the primary composite event
| Variable | Univariate regression | Multivariate regression | ||||||
|---|---|---|---|---|---|---|---|---|
| ß | OR | CI95 | p-value | ß | OR | CI95 | p-value | |
| Infrapatellar | 0.74 | 2.1 | 1.15-3.98 | 0.019 | 0.80 | 2.22 | 1.05-4.81 | 0.038 |
| Diabetes | 0.49 | 1.64 | 0.88-3 | 0.116 | 0.02 | 1.02 | 0.46-2.20 | 0.944 |
| Tobacco consumption | −0.14 | 0.87 | 0.37-1.95 | 0.742 | 0.56 | 1.75 | 0.61-4.88 | 0.285 |
| Male sex | −0.51 | 0.6 | 0.33-1.1 | 0.096 | −0.95 | 0.39 | 0.17-0.83 | 0.016 |
| Age | −0.02 | 0.98 | 0.95-1 | 0.086 | −0.03 | 0.97 | 0.94-1 | 0.068 |
| CLTI events | 1.43 | 4.17 | 2.26-7.78 | < 0.001 | 1.27 | 3.57 | 1.65-7.9 | 0.001 |
| AI events | −0.31 | 0.73 | 0.27-1.72 | 0.506 | 0.75 | 2.12 | 0.65-6.39 | 0.188 |
| Hematocrit | −0.13 | 0.88 | 0.83-0.92 | < 0.001 | −0.08 | 0.92 | 0.86-0.98 | 0.013 |
| Creatinine | 0.28 | 1.33 | 1.1-1.63 | 0.004 | 0.18 | 1.20 | 0.96-1.54 | 0.118 |
| Surgical revascularization | 0.03 | 1 | 0.43-2.12 | 0.993 | −0.20 | 0.82 | 0.31-1.97 | 0.666 |
AI: acute ischemia; CI: confidence interval; CLTI: chronic limb threatening ischemia; OR: odds ratio.
Table 4 Multivariate, regarding the primary composite event
| Variable | Multivariate regression | |||
|---|---|---|---|---|
| ß | OR | CI95 | p-value | |
| Infrapatellar | 0.84 | 2.33 | 1.00-5.79 | 0.030 |
| Diabetes | 0.09 | 1.09 | 0.48-2.38 | 0.814 |
| Tobacco consumption | 0.52 | 1.68 | 0.59-4.72 | 0.322 |
| Male sex | −0.94 | 0.39 | 0.17-0.83 | 0.017 |
| Age | −0.03 | 0.97 | 0.94-1 | 0.068 |
| CLTI events | 1.30 | 3.68 | 1.69-8.2 | 0.001 |
| AI events | 0.77 | 2.16 | 0.66-6.49 | 0.176 |
| Hematocrit | −0.08 | 0.92 | 0.86-0.98 | 0.015 |
| Creatinine | 0.18 | 1.20 | 0.96-1.54 | 0.122 |
| Surgical revascularization | −0.20 | 0.81 | 0.31-1.97 | 0.655 |
| Pure suprapatellar affection | 0.35 | 1.43 | 0.50-4.42 | 0.514 |
AI: acute ischemia; CI: confidence interval; CLTI: chronic limb threatening ischemia; OR: odds ratio.
Discussion
In this retrospective cohort study, the risk of developing the primary outcome (a composite of hospitalization of CLTI events and major amputations) was higher in the infrapatellar group than in the suprapatellar group. This higher risk was independent of baseline covariates that were thought to be clinically relevant in the prognosis of the patients and that were thought to confound the results. Further, we found an increased risk of developing minor amputation events in the infrapatellar group. On the other hand, risk of death, MI, stroke, or MACE was not different between both groups.
PAD is a worldwide pathology, affecting millions of patients with a yearly increasing incidence17,18. Understanding prognostic factors is of paramount importance to provide better pharmacological and revascularization procedures. The previous classifications systems have made focus in the severity of the symptoms (such as Rutherford and Fontaine classification)19, as well as the complexity of the lesions (such as the Trans-Atlantic Inter-Society Consensus [TASC] classification)20, with few mentions to the location of the disease itself. Aboyan et al. reported a poorer general prognosis of patients with proximal (aortoiliac) PAD compared with those with more distal PAD, independent of risk factors and comorbidities5. Our data collides with these results, but differences in sample population may explain these conflicting data. We selected a very comorbid population, as for inclusion criteria, the patients had to have symptomatic revascularization events to be considered for analysis. We found that frequently PAD affects multiple territories, but those patients with infrapatellar disease had worse prognosis than those with suprapatellar compromise.
Another significant mention is the rate of major amputations in our population. Our rates of major amputations are higher than in previous reports21,22. These differences may be explained by our older and more comorbid (i.e., higher prevalence of diabetes, atrial fibrillation, and previous stroke) population. In fact, we believe that our data reflects the actual condition of vascular patients in those countries in which an increasing aging population with a larger life expectancy.
Minor amputations events were also more prevalent in the infrapatellar disease group. Considered as a therapeutic goal, better delimitation of ulcer wounds reflect an ability to control diffuse ischemia in the ankles and feet. This improvement in delimitation may be due to the advances done in the endovascular revascularization technology that shifted the paradigm towards an endovascular first therapy, performing surgical procedures in those patients in which endovascular therapy failed. In our study, we have seen this shift as most of the revascularization procedures has been done percutaneously (13% in infrapatellar PAD and 20.7% in suprapatellar PAD).
Mortality, MI, and stroke (and subsequently MACE events) were similar in both suprapatellar and infrapatellar patients. Our event rates regarding these outcomes are in concord with previous literature21, nevertheless Aboyan et al. found higher mortality and cardiovascular events in proximal aortoiliac lesions5.
Diabetes (associated with a more advanced PAD)18,23 and CLTI frequently affect infrapatellar regions, thereby impairing worse prognosis in infrapatellar disease patients, independently from the location disease. A 3-fold increase in major amputation events in diabetic patients may be in fact due to a macro and microvascular damage in PAD patients24. Due to these confounding factors (among others), we performed a multivariate regression analysis to account for these covariates, and found impact of infrapatellar disease independently of the covariates included in the model.
Other hypothesis may explain these findings. First, infrapatellar disease may have a more prolonged course to become symptomatic, with patients consulting upon limb ulceration emerge, making the prognosis in these patients poorer. On the other hand, proximal aortoiliac lesions generally impair intermittent claudication symptoms, which frequently impair quality of life to the patients, warranting a prompt medical consult. Second, revascularization of infrapatellar stenosis may be more challenging due to smaller vessel size, increased prevalence of chronic occlusions, more calcified stenosis, requiring more aggressive therapies compared to suprapatellar disease. Third, we found an increased prevalence of acute ischemia in the suprapatellar population. These patients generally have a worse prognosis that IC patients, and the risk increases by delay in revascularization. Due to the retrospective nature of our work, we could not account feasibly the time to reperfusion in these patients. Nevertheless, we acknowledge that clinical presentation (i.e., CLTI and acute ischemia) may impair worse prognosis, this is the reason which we included acute ischemia and CLTI in the multivariate regression analysis to account for this factor.
Limitations
The retrospective nature of our study which may impair unknown bias that is responsible for the findings and may impair the external validity of the data, such as improvement in the Rutherford classification or improvement in wound care. Second, the small sample size and small number of events limits the number of variables which can be fitted in the multivariate model, with the risk of eventual over fitting of the model. Third, we could not account for the data which are included in the recent WIFI classification (such as wound characteristics, persistent infection, and ongoing ischemia) nor the number of vessels affected in the infrapatellar region25. Fourth, we used broad inclusion and exclusion criteria, including patients with diverse clinical presentation (i.e., intermittent claudication, chronic threatening limb ischemia, and acute ischemia) with diverse clinical evolution, which may affect the interpretation of our findings. Further research in these diverse clinical presentations would yield interesting information regarding the impact of infrapatellar disease.











 nueva página del texto (beta)
nueva página del texto (beta)