Introduction
Evolutionary biology is central to understanding the causes of health problems1,2, including cardiovascular diseases. Many cardiovascular ailments and their risk factors are linked to the mechanisms that explain evolutionary medicine which include antagonistic pleiotropy, ecological antagonistic pleiotropy, atavisms and heterochrony. In this review we analyze each mechanism and show examples of their application in experimental medicine. Pleiotropism refers to genes participating in different functions while antagonistic pleiotropism refers to genes that are beneficial during certain stages of development but become detrimental in others. A poor adaptation to the current environment and lifestyle is known as antagosistic pleiotropy or ecological antagonistic pleiotropy3,4. Congestive heart failure, hypertension and atherosclerosis may result from ecological antagonistic pleiotropy. Atavistic genes or conditions are characteristics that were expressed in our ancestors but have remained silent during evolution being suddenly expressed without an apparent cause during the appearance of a disease. It also refers to situations that were present early in development and reappear suddenly and an example of them could be the change in the metabolism of the heart from fatty acid dependent to glucose dependent in hypoxic conditions such as a stroke. Heterochrony is the expression of genes that cause the appearance of traits at a different timing during development and is therefore related to atavisms3,5.
It was not until 1994 that a proper anchorage of evolutionary biology to medicine was reached when doctor Randolph M. Neese and evolutionary biologist George C. Williams, published their book “Why we get sick” and novel area of study, known as “Darwinian Medicine” was proposed6. Also recently, information of developmental biology has been added to explain some aspects of Darwinian medicine after a new field of research that takes into account evolutionary developmental biology evolved, which is known as Evo-Devo (figure 1). The approach of the origin of the diseases being determined in the early stages of development had been previously addressed by the “life history” theory of Ronald A. Fisher. This author, in his work “The Genetical Theory of Natural Selection” in1930, proposed the concept of “trade-off” (commitment or investment exchange)7. This concept explains that there exists a limited amount of resources (energy, nutrients or time) which must be administered between different activities in the individual, determining lifespan and susceptibility to diseases. Even before a baby is born, the maternal environment is critical for fetal development and the expression of genes in terms of resources7. According to these new fields of research, diseases, including cardiovascular ailments, are considered as phenotypes generated by the expression of sets of genes during development8 in a determined environment which are then subject to natural selection9. The genetic material, the environment or an interaction between both may be the cause of diseases, including those of the cardiovascular system10-12.
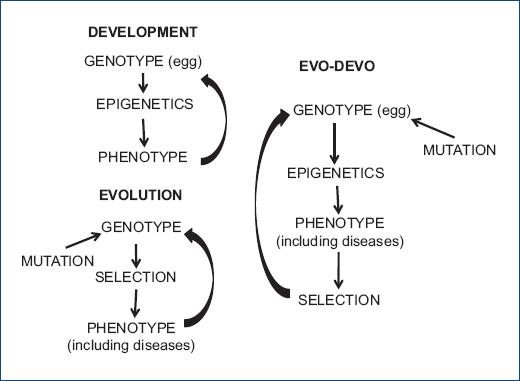
Figure 1 Evo-Devo, the new field of research that takes into account the influence of evolutionary concepts in developmental biology.
Mechanisms involved in evolutionary medicine, diseases that may be caused by these mechanisms, particularly in cardiovascular illnesses, and some possible experimental approaches that can be used in experimental medicine based on these mechanisms are illustrated in Table 1. Moreover, infectious diseases and the recent epidemic of COVID-19 where the cardiovascular system is compromised are associated to microbial pathogenesis and the ecology and evolution of viruses and microorganisms13. Pathogenesis which is the capacity of microorganisms to cause damage in a host organism14 is also studied by evolutionary medicine. Therefore, the increased knowledge of evolutionary medicine and its mechanisms is important to understand cardiovascular problems and the current and future pandemics that may affect the cardiovascular system.
Table 1 Examples of conditions in which humans evolved and later changed, leading to mis- adaptation and an increased risk to develop diseases
| Condition to which humans are not adapted | Environmental conditions to which adaptation occurred | Diseases to which RISK increased |
|---|---|---|
| Diet high in salt | Little salt in the diet | Hypertension |
| Abundant diet high in carbohydrates | Famine and necessity to store fat | Increased risk of obesity, Insulin Resistance, Metabolic Syndrome and type-2 Diabetes |
| Sedentarism | High physical activity | Insulin Resistance, Obesity, Metabolic Syndrome and type-2 Diabetes |
| Longevity | Shorter lifespan | Increased exposure to the above mentioned risk factors |
Pleiotropy in cardiovascular diseases
Pleiotropy is the property of genes to affect multiple functions or characters of an organism and it was first noticed 100 years ago when mutations were found to affect more than one phenotypic characteristic15. Some hormonal agents that are involved in the regulation of the cardiovascular system, such as the natriuretic peptides (NP)s and the rennin-angiotensin-aldosterone system (RAAS), are highly peliotropic. The NP system and particularly the atrial NP is a cardiac hormone with cardiovascular, renal and metabolic properties16. The NP are genetically different but are related functionally and structurally and they are secreted by cardiomyocytes, fibroblasts, endothelial cells, immune cells and immature cells. In fact, the ANP and BNP are encoded by the same precursor. NPs are mainly produced in response to wall stretch and they protect from natriuresis, diuresis, vasodilation, antiproliferation, antihypertrophy and antifibrosis. NPs also constitute a compensatory mechanism against elements from the RAAS and sympathetic nervous system. Moreover, NPs play a central role in the regulation of heart failure (HF)17.
On the other hand, the RAAS mediators have pleiotropic properties including the improvement of cognition. Different RAS components promote neuroprotection against diseases such as Alzheimer. Moreover, there exists a crosstalk between the RAAS and other systems such as the cholinergic, dopaminergic and adrenergic systems18.
Pleiotropic genes underlie genetic covariance between traits, often causing evolutionary constraints. Genes vary widely in their degree of pleiotropy, but this variation is often considered as a by-product of their evolutionary history19. The finding of pleiotropy has major implications for the evolution of complex organisms and the mapping of mutations that cause diseases 15.
Whether genes become pleiotropic or specialize on a single function depends on the nature of trade-offs as gene activities contribute to different traits and on how the functionality of these traits affects fitness. When a gene product can perform well at two functions, it evolves to do so, but it does not evolve when pleiotropy would greatly disrupt each function.Even when pleiotropy does evolve, not all genes are expected to become equally pleiotropic. Genes with higher levels of expression are more likely to evolve greater pleiotropy. In many cases, genes duplicate when they develop pleiotropy. Duplicates are expected to maintain a certain degree of functional redundancy, with the gene contributing more to the trait with the most important functionality19.
Antagonistic pleiotropy and cardiovascular diseases
Antagonistic pleiotropy, or genetic trade-off, is frequently invoked in theories of aging, cancer, genetic disease and other common phenomena important to Darwinian medicine20. An adaptive change in one character at a certain stage can be associated with deleterious pleiotropy later in life21,22. The concept of a Darwinian-evolutionary basis for the development of diseases related to age postulates that genetic traits that are beneficial in younger years to allow for successful reproduction may become deleterious in the elderly when selective pressure is not effective anymore. Diseases associated to antagonistic pleiotropy include atherosclerosis, benign and malignant prostate hypertrophy, Alzheimer's disease and the reciprocal relationship between cellular senescence and cancer23. In fact, as an example of antagonistic pleiotropy in the cardiovascular system, the RAAS has been implied to participate in cancer in the elderly population24. The prevalence of antagonistic pleiotropy, the genes selected, and to what extent and how its undesirable effects can be resolved remains unclear20.
Evolutionary pressures have selected for successful reproduction, making it likely that the post-reproductive physiology is an epigenetic and pleiotropic manifestation of the optimization for early fitness. Cellular senescence and aging are antagonistically pleiotropic manifestations of evolutionary pressures to prevent malignant transformation, and therefore, aging may be the price we pay to avoid cancer. The beneficial paradox may be that the maximum lifespan potential of humans may have been achieved, in part, due to our ability to grow old25. Furthermore, aging which is also a late acquisition in the evolution of the human species, has brought with it an enormous increase in cardiovascular diseases and the related risk factors26.
Ecological antagonistic pleiotropy
Many diseases are generated by a poor adaptation to current environmental conditions that differ from those in which humans evolved, thus being the result of ecological antagonistic pleiotropy. This causes a mismatch between our evolutionary constitution and our nowadays conditions of life rendering us more vulnerable to diseases. Therefore, there seems to be an ecological antagonistic pleiotropy effect upon evolutionary traits that predisposes our species to many diseases that are becoming epidemics in the XXI century. When viewed from the perspective of evolutionary biology a thrifty and a pro inflammatory condition could underlie the appearance of atherosclerosis and other cardiovascular diseases constituting an example of ecological antagonistic pleiotropism. During the Miocene and Pleistocene periods, evolutionary pressures selected for subjects with a large part of the genome codifying for innate immunity responses and inflammation that helped in the fight against infections and trauma survival27.
Insulin resistance is accompanied by an increase in the synthesis of insulin (hyperinsulinemia) as a compensatory mechanism. The acquisition of insulin resistance was a key event to survive famine periods in ancient times. Some tissues such as skeletal muscel develop resistance to insulin, while other cells are still highly responsive to insulin. Since many cells have a limited capacity to utilize glucose, the remaining sugar was stored as fat. Genes participating in the storage of nutrient were also selected favoring individuals who performed gluconeogenesis and were resistant to insulin. The thrifty genotype allowed for the survive of long fasting situations, but nowadays, it may bring negative consequences since it is linked to the development of obesity or type 2 diabetes28. Moreover, insulin resistance may also alter processes such as aging, reproduction, immunity and brain functions29.
Our hunter-gatherer ancestors did not develop atherosclerosis and their lifestyle was characterized by large periods of physical activity and a diet rich in protein. In our nowadays lifestyle, the genes with which we evolved have been turned into risk factors for cardiovascular diseases26,27. Congestive cardiac failure and hypertension also result from our ancestral adaptation to gravitation and bipedalism and an environment poor in salt and water which resulted in neurohumoral mechanisms adapted to a terrestrial lifestyle 500,000 years ago30. In fact, hypertension is present in giraffes since their brain is in need of a mechanisms to receive and adequate supply of blood against gravitation due to their long neck31,32. Metabolic Syndrome and obesity that are important risk factors to develop cardiovascular diseases could be the result of our current feeding habits and sedentary lifestyle. Some diseases that may be the result of this pleiotropy are shown in Table 2 and include Metabolic Syndrome, obesity and diabetes that increase the risk to develop cardiovascular diseases.
Table 2 Mechanisms involved in Darwininan medicine with examples of their participation in different diseases. Experimental models based in the Darwinian medicine mechanisms
| Mechanisms involved in darwininan medicine | Diseases | Experimental models in which research has been done |
|---|---|---|
| – Atavistic traits | – Heart hipoxia and ischemia where metabolism changes
from fatty acid dependent to glucose dependent. – Heart regenerative capacity – Cancer |
– Role of stem cells in tissue reparation – Change in metabolism in the hypoxic heart |
| – Heterochrony (neoteny) | – Congenital malformations of the heart where there is
intercommunication between the atria or ventricles – Psyhiatric and neurological diseases (Attention deficit disorder and hyperactivity) |
– Regeneration in the axolotl |
| – Antagonistic pleiotropy – Ecological antagonistic pleiotropy |
– Atherosclerosis – Congestive heart failure – Hypertension – Aging – Metabolic syndrome – Obesity – Diabetes |
– Aging of the cardiovascular system – Vascular responses in metabolic syndrome – Early programming of hypertension |
Experimental conditions in which ecological antagonistic pleiotropy is approached
Several experimental models in which diet is changed simulate feeding alterations or our current lifestyle contrasting to the conditions in which we evolved. Consequences of changes in diet during the adult stage or during development have been studied33.
A Metabolic Syndrome rat model in which sucrose at a high concentration such as is present in soda beverages is administered since weaning rats has been developed34. Rats become obese, hypertense, hypertriglyceridemic and they show insulin resistance35. The long- lasting effects of changes in the diet during short periods of time during development that lead to programming of diseases during adulthood have also been studied36-37. A change in the diet to a sucrose rich diet during a critical window of development around weaning has been studied and it was found that rats are programmed by this change in the diet to develop hypertension when they reach the adult stage38,39. The acceleration of aging, particularly at the level of the vessels, when Metabolic Syndrome is present in rats has also been studied40.
Atavistic genes and heterochrony
Characteristics phylogenetically or developmentally remote, belonging to organisms of earlier appearance in evolution or to earlier stages of development that are suddenly shown during the development of a disease are considered as atavisms41. Atavistic genes are fragments of DNA whose expression seemed to have disappeared from the development of the organism and that appear suddenly during its life course. The first studies of Evo-Devo identified conserved sets of toolkits common to most metazoans which include atavistic genes42. Atavistic traits may be a characteristic or a cause of diseases and are important mechanism in Darwinian medicine43.
The change in the heart metabolism form dependent on fatty acids to dependent on glucose of under hypoxic conditions44,45 could constitute an atavistic trait that is suddenly brought about in hypoxic conditions such as a stroke. During ontogeny, the fetal and neonatal heart oxidizes glucose easier than the adult heart. Glucose is obtained first by simple diffusion and its transport is limited by its utilization rate. However, later, glucose enters cardiac muscle cells through a facilitated diffusion process and it is at this time that transport begins to be regulated by a variety of factors46. During the perinatal period, myocardial metabolism changes from a carbohydrate-based system as an energy source with anaerobic capacity to an aerobic metabolism, using free fatty acids as a substrate47,48. Furthermore, glucose is not the only carbohydrate that is largely consumed by the developing heart, but a large amount of lactate is also consumed49.
In a similar way as in ontogeny, during the evolutionary development of the heart, a trend towards greater dependence on oxidative metabolism is observed, indicating that the most primitive cardiac muscles were less dependent on oxygen. The most efficient machinery for the use of carbohydrates is found in hearts that depend on anaerobic metabolism, and perhaps even in some primitive vertebrates. However, when the contractility and strength requirements of the hearts were exceeded, hearts began to develop another type of metabolism and expanded their ability to obtain energy from fatty acids50. These evolutionary changes could explain why anaerobic glycolysis is maintained in amphibians such as the axolotl and the frogs and is lost in the mammals. Furthermore, in many current organisms the metabolism of the heart is still capable of changing from fatty acid dependent to carbohydrate dependent under hypoxic conditions in a similar way as in abnormal hypoxic conditions in vertebrates. This occurs in some vertebrates that dive in the water such as lungfish51, in turtles52, in Weddell seals53 and during the flight and flutter of hummingbirds54. The work of the heart during diving in seals is maintained by oxidative metabolism and lactate is the preferred substrate53.
The metabolic change found during ontogeny results in an accumulation of reactive oxygen species (ROS), which activates the DNA damage response pathway, resulting in permanent cardiac myocyte cell cycle arrest. As a consequence of changes in oxygen levels in the postnatal environment, a permanent arrest of the cardiomyocyte cell cycle occurs, contributing to the loss of the regenerative capacity. Postnatal hypoxemia, ROS clearance, or inhibition of the DNA damage response pathway may prolong the proliferative capacity of cardiomyocytes, which could result in an enhanced regenerative response beyond the first week of life in mammals55. Atavisms seem to violate one of the central evolutionary principles, known as the Dollo's law. This law states that “an organism is unable to return, even partially, to a previous stage already reached in the ranks of its ancestors.” Although it is still not clear what triggers and controls the reactivation of dormant traits, atavisms are a challenge to evolutionary biologists and geneticists56.
The participation of conserved atavistic genes in essential functions in primitive organisms and their possible functionality before the appearance of multicellular species illustrates their importance. Some atavistic genes are considered to be proto-oncogenes. The return to the functionality of certain genes by defective regulation in modern organisms might be involved in the appearance of diseases such as cancer57. This is consistent with current understanding of cancer and explains the paradoxical rapidity with which cancer acquires a suite of mutually-supportive complex abilities57. Comparative genomics and the phylogeny of basal metazoans should help identify relevant genes associated to atavisms in mammals and yield the order in which they evolved.
Other diseases in which atavistic conditions have been described to play a role include pulmonary fibrosis58. Abnormal recapitulation of developmental pathways and epigenetic changes link idiopathic pulmonary fibrosis with ageing and aberrant epithelial activation67. Atavistic traits have also been associated to neurological diseases such as attention deficit disorder and hyperactivity59 and Down syndrome.
When genes or traits appear with a different timing during development, the process is known as heterochrony. Changes in timing in development were identified since Haekel60. Heterochrony constitutes a mechanism by which new gene expression patterns can produce novel morphological structures or can form robust patterns that can both facilitate and resist change. Heterochrony could be explained by the timing of appearance of modular structures of trait development and epigenetic interactions among modules during ontogeny which affect patterns of phenotypic and genetic variation61. Genetic modules include gene networks and gene cascades that link the genotype with cellular modules that comprise germ layers, embryonic fields or cellular condensations. Epigenetic processes such as embryonic inductions, tissue interactions, and functional integration, link morphogenetic units to the phenotype62. Any genetic change in the mapping of modules is reflected by a change in development21,22.
Heterochrony includes three patterns: a) neoteny, b) progenesis, and c) direct development. Neoteny or the appearance of the capacity to reproduce while the somatic development of the organism has not been reached could be the most and well-known form of heterochrony63.
An example of interest in cardiovascular diseases is found in the congenital malformations of the heart where communication between the chambers (atria or ventricles) persists in infancy or the adult life. These communications are found early in the embryololgical development and in other phylogenetcally related vertebrates with hearts consisting of 3 or 2 chambers64. Another example of heterochrony in the human population is the consideration of prolonged youthfulness or child-like behavior as advantageous for modern life with the consequent retention of characteristic behaviors and attitudes of earlier developmental stages for longer periods of time59.
Experimental conditions in which atavistic conditions or heterochrony are approached
Regarding experiments in relation with atavistic conditions or heterochrony, several diseases show traits or conditions that were present at earlier stages of life. It has been found that the mechanical and electric activity of the fetal and newborn hearts depends on glucose and fatty acid concentrations in the environment, being adapted to the conditions that are present at the different stages65. We have also worked on the change of metabolism in the adult heart under hypoxia and described the participation of the glucose transporters (GLUTs)66. As a possible application of this knowledge to practical medicine we studied the effect of the polarizing solution on the hypoxic heart and on coronary artery vasoconstriction. Benefic effects of the polarizing solution were found and under hypoxic conditions, coronary arteries relax in the presence of the polarizing solution which contrasts with the vasoconstrictive effect of hypoxic femoral arteries. This response of coronary arteries allows for a better perfusion of the myocardium67.
Regarding experimental work dealing with heterochrony studies have been done on the regeneration of the heart in a neotenic species, the axolotl. In this species the cardiomyocytes are able to regenerate and there is a functional recovery of this organ68,69.
On the other hand, when the differentiation stage of certain cells or organisms does not correspond to the environment in which they finally develop and live, a predisposition to diseases exists or a difficult situation arises to apply methodologies such as the use of stem cells for regeneration. A mismatch between the developmental stage of stem cells and the tissue in which they are implanted, that constitutes the environment to which they must adapt, is also an important issue for regenerative and Darwinian medicine3.
Infectious diseases affecting the cardiovascular system and evolutionary medicine
Infectious diseases related to cardiovascular consequences such as rheumatic fever and aortic valvulopathy which are caused by a previous streptococcal infection are associated to microbial pathogenesis and the ecology and evolution microorganisms13 which is studied by evolutionary medicine. There are pathogenic sets of genes that are jointly transcribed in microorganisms and that codify for virulence factors70. Pathogens are in a constant fight to survive and natural selection is acting on both the infectious agents and the hosts. Microorganisms modify their environment to survive, but since their environment is constituted by the host, they alter the host`s vital functions. These alterations are expressed as a disease, thus generating a physiological response that may lead to death. During the disease process, an arms race is established in which an increased survival for one of the participants in the infectious interaction results in a decreased survival for the other.
A decreased incidence in their appearance of rheumatic fever and valvulopathy has been related to hygienic changes in the environment brought about by a decrease in poverty and malnutrition, as well as a decrease in overcrowding that helps in the transmission of the microorganism from one host to another71. Another factor is the decrease in the virulence of the pathogen. Antibiotics that cause the death of the pathogen for these diseases and for those caused by other pathogens are only a short- term solution since the microorganisms will evolve to become resistant. An improved success in the fight against these infectious diseases will include the survival of pathogenic agents as commensal microorganisms, forming part of the microbiome13.
Regarding the current epidemic of COVID-19, the disease manifestations are associated to endothelial dysfunction72, thrombotic problems73 and it affects the heart74. The SARSCoV-2 virus interacts with the Angiotensin-converting enzyme 2 (ACE2) receptor75 that forms part of the RAAS and the virus disrupts cardiac mitochondrial functions, as part of its mechanisms to survive76. The virus also causes alterations in plasma lipids and in the activity of desaturases in its attempt to modify the host´s metabolism to favor its survival77.
Therefore, infectious diseases where the cardiovascular system is compromised are linked to microbial pathogenesis and the ecology and evolution microorganisms13 and the study of evolutionary medicine and its mechanisms may help understand the current and future pandemics that may affect the cardiovascular system.
Conclusion
Cardiovascular diseases are phenotypes generated by the expression of genes in a determined environment and are the subject of evolutionary medicine. Antagonistic pleiotropy, ecological antagonistic pleiotropy, atavisms and heterochrony. Antagonistic pleiotropism and ecological antagonistic pleiotropy are the main mechanisms involved in evolutionary medicine and participate in the development of congestive heart failure, hypertension and atherosclerosis. The change in the heart metabolism from fatty acid dependent to glucose dependent can be considered as an atavistic condition that appears in the heart after a stroke and may underlie the difficulty of the crdiomyocytes to regenerate. Heterochrony is the expression of genes that cause the appearance of traits at a different timing during development and is therefore related to atavisms. Evolutionary medicine also explains the interactions of pathogens and the host in infectious diseases where the cardiac tissue becomes a target. Mechanisms involved in evolutionary medicine participate in the generation of diseases and may be approached experimentally. Therefore, experimental medicine can shed light to evolutionary medicine and the origin of many health problems.











 nueva página del texto (beta)
nueva página del texto (beta)


