Introduction
Heart transplant (HTx) is a therapeutic option in selected patients with advanced heart failure. In 2018, HTx in Colombia corresponded to 6.6% of all transplants1.
Colombian National Transplant Network divides national territory into six regions, of which five have HTx centers. Our hospital belongs to region 1, which is the largest in extension. All HTx centers in region 1 are located in Bogotá D.C city situated at 2,640 m above sea level (masl), being the city with the highest altitude in the world where this procedure is performed2. Region 1 encompasses territories at altitudes from 0 masl and some populations located at more than 3,000 masl. Some of the transplanted patients in this region live at low altitudes and move to Bogota for transplant surgery, remaining the first 3 months of the post-operative period (POP) in the city. The majority of transplanted patients live in the city or in near localities, and remains at high altitudes (> 2,500 masl) before, during and after HTx.
Clinical trials have shown the effect of height on cardiovascular and pulmonary systems. Changes start from 1500 masl and are accentuated at higher altitudes. In general, high altitudes lead to pulmonary vasoconstriction and as a consequence, increase in pulmonary pressures and decrease in oxygen supply, favoring cardiovascular performance changes3. In HTx candidates, pulmonary hypertension (PH) is a poor prognostic factor, which carries an increased mortality risk and heart dysfunction4.
Due to effects of altitude on pulmonary physiology, it is presumed that transplant patients residing at higher altitudes may have a poor outcome after HTx, possibly by greater right ventricle (RV) dysfunction. Nowadays, no data have been published describing the hemodynamic behavior of RV in HTx patients at more than 2,500 masl.
The purpose of this study is to describe the RV pressures and systolic function, asses by transthoracic echocardiogram (TTE) in a historical cohort of HTx patients in our hospital. The measurements analyzed correspond to the immediate POP (Days 1-7 post-HTx), 3, 6, 12, and 24 months after HTx, both in patients living at an altitude of 2,640 masl, and in those living at a lower altitude.
Methods
Study design
Historical cohort study of patients undergoing HTx in a high complexity health-care center in Bogotá, Colombia.
Population
Patients older than 18 years-old diagnosed with stage D heart failure who underwent HTx between November 2005 and May 2019 at Fundación Cardioinfantil – Instituto de Cardiología in Bogotá, Colombia. Patients transplanted in other institutions were excluded from the study.
The protocol was approved by the institutional ethics committee.
Data collection
Patients were selected from the Heart Failure and Transplant group data base. Socio-demographic data, heart failure etiology, pre-transplant evaluation, and donor data were extracted from the institutional electronic medical records. Patient identification data anonymity was guaranteed.
Transthoracic echocardiograms (TTE)
All pre- and post-HTx studies were performed at our hospital. TTE were performed using Phillips iE33 (Koninklijke Philips n.v.), Phillips EPIQ7 (Koninklijke Philips n.v.), and GE VividE9 (General Electric Company) equipment. The study data were extracted from the medical records.
As a result of the retrospective and historical nature of the evaluated cohort, data were taken from the echocardiographic parameters available on the clinical records. All studies were performed by experienced cardiologists trained in echocardiography (board certified > 5 years). According to the physician criteria (“eyeball” estimation), the RV function was classify as normal, mild, moderate, or severely reduced. In addition, the classification was supported by the Tricuspid annular plane systolic excursion (TAPSE) as follows: 12-16 mm mild dysfunction, 8-11 mm moderate dysfunction, and < 8 mm severe dysfunction. The systolic pulmonary artery pressure (sPAP) and inferior vena cava diameter were registered if available.
A second evaluation of images performed in patients transplanted between January 2018 and May 2019 including every echocardiographic parameters was done by a different physician with more than 5 years of experience in echocardiography. RV fractional area change (RVFAC) was also measured and a value of < 35% was considered abnormal as stated by ASE guidelines5. These RV function measurements were compared with the qualitative evaluation found in the patients echo reports, mentioned previously.
Analysis
Extreme data, missing or non-concordant values auditory was performed. A descriptive analysis of dependent and independent variables was performed. Continuous variables were described after Shapiro–Wilk test of normality using means, standard deviation or median, and interquartile range. The ordinal or nominal categorical variables were described in absolute proportions and frequencies.
The delta between echocardiographic variables was determined with ANOVA repeated measures for normal distribution variables. In nonparametric distribution variables, Wilcoxon rank-sum test was used.
Data obtained from the second TTE analysis of HTx patients between January 2018 and May 2019 were used for a concordance study and differences tests.
All analyzes were performed on STATA14, licensed by the researchers.
Results
General characteristics
Ninety-one patients were transplanted at our institution between April 2005, and May 2019. Table 1 describes general characteristics. Most of the patients were men with a mean age of 47 years old. The main heart failure etiologies were as follows: dilated (idiopathic) (n = 31-36.5%), ischemic (n = 26-30, 2%), and chagasic (n = 14-16, 3%). Most frequent pre-transplant comorbidities were hypertension, diabetes, and smoking. Two thirds of the population presented chronic kidney disease classified as Stages 2 and 3 at time of HTx. Most of the patients (61%) lived at more than 2,500 masl (Table 1).
Table 1 Population general characteristics
| Characteristic | < 2,500 masl (n = 27) | ≥ 2,500 masl (n = 58) | No data (n = 6) | Total (n = 91) |
|---|---|---|---|---|
| Age at transplant in years, median (IQR) | 44 (38 - 54) | 47 (37 - 58) | 51,5 (48 - 56) | 47 (38 - 56) |
| Male, n. (%) | 21 (77.78) | 44 (75.86) | 5 (83.33) | 70 (76.92) |
| Follow-up time in months, median (IQR) | 83.71 (57.13 - 115.3) | 68.66 (30.43 - 129.73) | 36.83 (34 - 50.73) | 69.2 (34 - 119) |
| Past medical history | (n = 20) | (n = 36) | (n = 1) | (n = 57) |
| Arterial hypertension, n. (%) | 2 (10) | 5 (13.89) | 1 (100) | 8 (14.03) |
| Type 2 diabetes, n. (%) | 1 (5) | 5 (13.89) | 1 (100) | 7 (12.28) |
| Smoking, n. (%) | 1 (5) | 4 (11.11) | 1 (100) | 6 (10.53) |
| Dyslipidemia, n. (%) | 1 (5) | 4 (11.11) | 0 (0) | 5 (8.77) |
| COPD, n. (%) | 1 (5) | 2 (5.56) | 0 (0) | 3 (5.26) |
| Asthma, n. (%) | 0 (0) | 1 (2.78) | 0 (0) | 1 (1.75) |
| Pulmonary embolism, n. (%) | 0 (0) | 1 (2.78) | 0 (0) | 1 (1.75) |
| Cancer, n. (%) | 1 (5) | 0 (0) | 0 (0) | 1 (1.75) |
| Chronic kidney disease staging | (n = 20) | (n = 35) | (n = 1) | (n = 56) |
| No renal disease, n. (%) | 0 (0) | 2 (5.71) | 1 (100) | 3 (5.36) |
| Stage 1, n. (%) | 7 (35) | 10 (28.57) | 0 (0) | 17 (30.36) |
| Stage 2, n. (%) | 7 (35) | 16 (45.71) | 0 (0) | 23 (41.07) |
| Stage 3A, n. (%) | 5 (25) | 6 (17.14) | 0 (0) | 11 (19.64) |
| Stage 3B, n. (%) | 1 (5) | 0 (0) | 0 (0) | 1 (1.79) |
| Stage 4, n. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Stage 5, n. (%) | 0 (0) | 1 (2.86) | 0 (0) | 1 (1.79) |
| Heart failure etiology | (n = 26) | (n = 56) | (n = 4) | (n = 86) |
| Dilated (Idiopathic), n. (%) | 9 (34.61) | 20 (35.71) | 2 (50) | 31 (36.05) |
| Ischemic, n. (%) | 7 (26.92) | 18 (32.14) | 1 (25) | 26 (30.23) |
| Chagasic, n. (%) | 6 (23.08) | 8 (14.29) | 0 (0) | 14 (16.28) |
| Congenital, n. (%) | 2 (7.69) | 2 (3.57) | 0 (0) | 4 (4.65) |
| Chemotherapy, n. (%) | 2 (7.69) | 1 (1.79) | 0 (0) | 3 (3.49) |
| Myocarditis, n. (%) | 0 (0) | 2 (3.57) | 0 (0) | 2 (2.32) |
| Peripartum, n. (%) | 0 (0) | 2 (3.57) | 0 (0) | 2 (2.32) |
| Hypertrophic, n. (%) | 0 (0) | 1 (1.79) | 1 (25) | 2 (2.32) |
| Valvular, n. (%) | 0 (0) | 1 (1.79) | 0 (0) | 1 (1.16) |
| Noncompact heart disease, n. (%) | 0 (0) | 1 (1.79) | 0 (0) | 1 (1.16) |
| Patient status | (n = 27) | (n = 58) | (n = 6) | (n = 91) |
| Alive, n. (%) | 23 (85.19) | 44 (75.86) | 4 (66.67) | 71 (78.02) |
| Death, n. (%) | 4 (14.81) | 14 (24.14) | 2 (33.33) | 20 (21.98) |
| Dead within 30 days, n. (%) | 2 (7.4) | 4 (6.89) | 1 | 7 (7.69) |
| Severe RV dysfunction death, n. (%) | 1 (3.71) | 2 (3.44) | 0 (0) | 3 (3.29) |
COPD: chronic obstructive pulmonary disease; n: number; %: percentage; IQR: Interquartile range.
Follow-up was 14 years, 30-day survival was 91.85% (95% CI: 84-96). Of seven patients who died in this period, three died within 48 h post-transplant, all due to severe RV dysfunction. Five-year survival was 78% (95% CI: 60-85). Late mortality was mainly infectious from lung and/or soft tissue (Table 1).
Pre-transplant receiver evaluation
In pre-transplant evaluation, all patients were eutrophic. INTERMACS classification was 5 or 6 in 68% of patients. All patients presented Post-capillary pulmonary hypertension (PH). Those with mPAP > 40 mmHg underwent vasoreactivity test with nitroprusside and/or prostaglandins. TTE sPAP was similar to that obtained by right catheterization. Panel-reactive antibody (PRA) I and II were negative (< 10%) in 80% and 76% of patients, respectively. Table 2 shows absolute values and percentages of variables described above.
Table 2 Recipient evaluation before transplant
| Antropometry | < 2,500 masl (n = 20) | ≥ 2,500 masl (n = 36) | Total (n = 57) |
|---|---|---|---|
| Weight in Kg, mean (SD) | 64.64 (14.68) | 66.18 (10.09) | 65.74 (11.74) |
| Height in cms, mean (SD) | 164 (7.9) | 166.86 (9.26) | 166.28 (8.75) |
| BMI, median (IQR) | 22.45 (20.45 - 25.8) | 23.25 (20.95 - 25.1) | 23.3 (20.8 - 25.5) |
| Right heart catheterization | (n = 18) | (n = 33) | (n = 52) |
| sPAP in mmHg, mean (SD) | 46.11 (9.22) | 47.18 (13.44) | 47.03 (11.91) |
| mPAP mmHg, mean (SD) | 32.72 (8.41) | 32.90 (9.29) | 32.82 (8.82) |
| GTP mmHg, Median (IQR) | 9 (6 - 16) | 10.5 (8.5 - 14.5) | 10 (7.5 - 15) |
| PVR Wood Units, Median (IQR) | 3.7 (2.9 - 4.72) | 3.3 (2 - 4.2) | 3.3 (2.5 - 4.4) |
| PCWP, mean (SD) | 22.38 (4.41) | 21.48 (6.65) | 21.73 (5.89) |
| Pulmonary reactivity test, YES, n. (%) | 8 (44.44) | 17 (51.51) | 26 (53.1) |
| Transthoraric echocardiogram | n = 16 | n = 26 | n = 42 |
| sPAP in mmHg, mean (SD) | 48.18 (9.36) | 50.71 (13.94) | 49.75 (12.33) |
| Mitral regurgitation classification | (n = 17) | (n = 29) | (n = 46) |
| Mild, n. (%) | 5 (29.41) | 9 (31.03) | 14 (30.43) |
| Moderate, n. (%) | 7 (41.18) | 10 (34.48) | 17 (36.96) |
| Severe, n. (%) | 5 (29.41) | 10 (34.48) | 15 (32.61) |
| INTERMACS | (n = 18) | (n = 32) | (n = 50) |
| 1, n. (%) | 1 (5.56) | 4 (12.50) | 5 (10) |
| 2, n. (%) | 0 (0) | 0 (0) | 0 (0) |
| 3, n. (%) | 2 (11.11) | 3 (9.38) | 5 (10) |
| 4, n. (%) | 3 (16.67) | 1 (3.12) | 4 (8) |
| 5, n. (%) | 7 (38.89) | 10 (31.25) | 17 (34) |
| 6, n. (%) | 5 (27.78) | 12 (37.50) | 17 (34) |
| 7, n. (%) | 0 (0) | 2 (6.25) | 2 (4) |
| Support | (n = 18) | (n = 32) | (n = 50) |
| Ventilatory support, n. (%) | 1 (5.56) | 4 (12.50) | 5 (10) |
| Mechanical circulatory support, n. (%) | , | 5 (15.62) | 7 (14) |
| Panel-reactive antibody | (n = 18) | (n = 32) | (n = 50) |
| PRA I, negative (<10%), n. (%) | 12 (66.67) | 28 (87.50) | 40 (80) |
| PRA II, negative (<10%), n. (%) | 13 (72.22) | 25 (78.12) | 38 (76) |
sPAP: systolic pulmonary artery pressure; mPAP: mean pulmonary artery pressure; TPG: transpulmonary gradient; PVR: pulmonary vascular resistance; PCWP: pulmonary capillary wedge pressure. TTE: transthoracic echocardiogram; PRA: panel-reactive antibody; n: number; %: percentage; IQR: Interquartile range; SD: standard deviation.
Donor characteristics
Most donors were men, aged under 35-years. Principal mortality cause was traumatic brain injury. Majority of donors (64%) lived at more than 2000 m above sea level. The main support required during preparation and studies before organ removal was vasopressor (62%) and a 26% also required inotropic support. No donor received amiodarone before organ removal (Table 3).
Table 3 Donor characteristics
| Characteristic | < 2,500 masl (n = 18) | ≥ 2,500 masl (n = 32) | Total (n = 50) |
|---|---|---|---|
| Age in years, median (IQR) | 28.5 (24 - 38) | 26 (19.5 - 34) | 27 (22 - 35) |
| Male, n, (%) | 14 (77.78) | 25 (78.12) | 39 (78) |
| Antropometry | (n = 17) | (n = 31) | (n = 48) |
| Weight in Kg, mean (SD) | 67.82 (10.77) | 71.93 (8.85) | 70.5 (9.7) |
| Height in cms, mean (SD) | 166.88 (9.01) | 168.71 (7.25) | 168.1 (7.9) |
| BMI, mean (SD) | 24.23 (2.55) | 25.34 (3.42) | 24.9 (3.2) |
| Death cause | (n = 18) | (n = 32) | (n = 50) |
| Ischemic stroke, n, (%) | 7 (38.89) | 6 (18.75) | 13 (26) |
| Intracranial hemorrhage, n, (%) | 0 (0) | 1 (3.12) | 1 (2) |
| Traumatic Brain Injury, n, (%) | 11 (61.11) | 25 (78.12) | 36 (72) |
| Altitud at residence | (n = 18) | (n = 32) | (n = 50) |
| < 1,000 masl, n, (%) | 1 (5.56) | 4 (12.5) | 5 (10) |
| 1,000 - 2,000 masl, n, (%) | 5 (27.78) | 8 (25) | 13 (26) |
| > 2,000 masl, n, (%) | 12 (66.67) | 20 (62.5) | 32 (64) |
| Support | (n = 18) | (n = 32) | (n = 50) |
| Vasopressor requirement, n, (%) | 11 (61.11) | 20 (62.5) | 31 (62) |
| Inotropic requirement, n, (%) | 8 (44.44) | 5 (15.62) | 13 (26) |
| Amiodarone use, n, (%) | 0 (0) | 0 (0) | 0 (0) |
n: number; %: percentage; IQR: Interquartile range; SD: standard deviation.
Organ ischemia time on average was 128-min (96-150 min), with a perfusion time of 150-min (80-206 min.).
Gender mismatch (female donor/male recipient) was identified in 11% of patients. None of transplanted patients presented a weight mismatch.
Echocardiographic post-operative features
Echocardiographic results were available in 37 patients (40.6%) (Table 4). Some degree of RV dysfunction was evidenced in the immediate post-operative period in 95% of HTx-patients (Mild 27%, moderate 43%, and severe 24%). There was a progressive reduction in the percentage of patients with RV systolic dysfunction: about 41% at 3th month (Reclassify as mild in 3% of the patients and, as moderate in 38%), 31% at 6th month (Reclassify as mild in the 3%, and as moderate in 29%), and 17% at 24th month (Reclassify as mild in 7%, and moderate in 10%). After the 3rd month, none of the patients presented severe RV dysfunction (Figure 1A). The analysis of RV systolic dysfunction after the 3rd month of HTx is shown in (Figure 1B).
Table 4 Echocardiographic characteristics
| Inmediate POP | 3 Months POP | 6 Months POP | 12 Months POP | 24 Months POP | p-value | |
|---|---|---|---|---|---|---|
| Culitative Evaluation | n = 37 | n = 32 | n = 31 | n = 30 | n = 29 | 0,0001 |
| Mild RV Disfunction, n (%) | 10 (27) | 12 (37.5) | 9 (29) | 5 (16.7) | 2 (6.9) | |
| Moderate RV Disfunction, n (%) | 16 (43.2) | 1 (3.1) | 1 (3.2) | 2 (6.7) | 3 (10.3) | |
| Severe RV Disfunction, n (%) | 9 (24.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Normal RV Function, n (%) | 2 (5.4) | 19 (59.4) | 21 (67.7) | 23 (76.7) | 24 (82.7) | |
| Cuantitative Evaluation | ||||||
| TAPSE (mm) mean ± SD | 8.9 ± 4.9 | 15.1 ± 3.6 | 13.5 ± 4.2 | 14.9 ± 2.8 | 15.8 ± 4.9 | 0,02 |
| sPAP (mmHg) mean ± SD | 39.8 ± 8.2 | 33.8 ± 7.7 | 31.1 ± 6.2 | 30.2 ± 6.4 | 31.0 ± 5.0 | 0,04 |
| IVC dilation (> 2,1 cms) n (%) | 11 (29.7) | 2 (6.2) | 0 (0) | 0 (0) | 0 (0) | NS |
| LVEF % mean ± SD | ND | 59.2 (6.7) | 57.8 (6.5) | 57.5 (5.1) | 58.5 (4.6) | 0,56 |
| Tricuspid Regurgitation | 0,005 | |||||
| Mild, n (%) | 19 (51.3) | 17 (53.1) | 16 (51.6) | 18 (60) | 18 (62) | |
| Moderate, n (%) | 8 (21.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Severe, n (%) | 3 (8.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Normal, n (%) | 7 (19.8) | 15 (46.9) | 15 (48.4) | 12 (40) | 11 (38) |
sPAP: systolic pulmonary artery pressure; TAPSE: Tricuspid annular plane systolic excursion; LVEF: Left ventricular ejection fraction; IVC: inferior vena cava; RV: right ventricle; n: number; %: percentage; IQR: Interquartile range; SD: standard deviation.
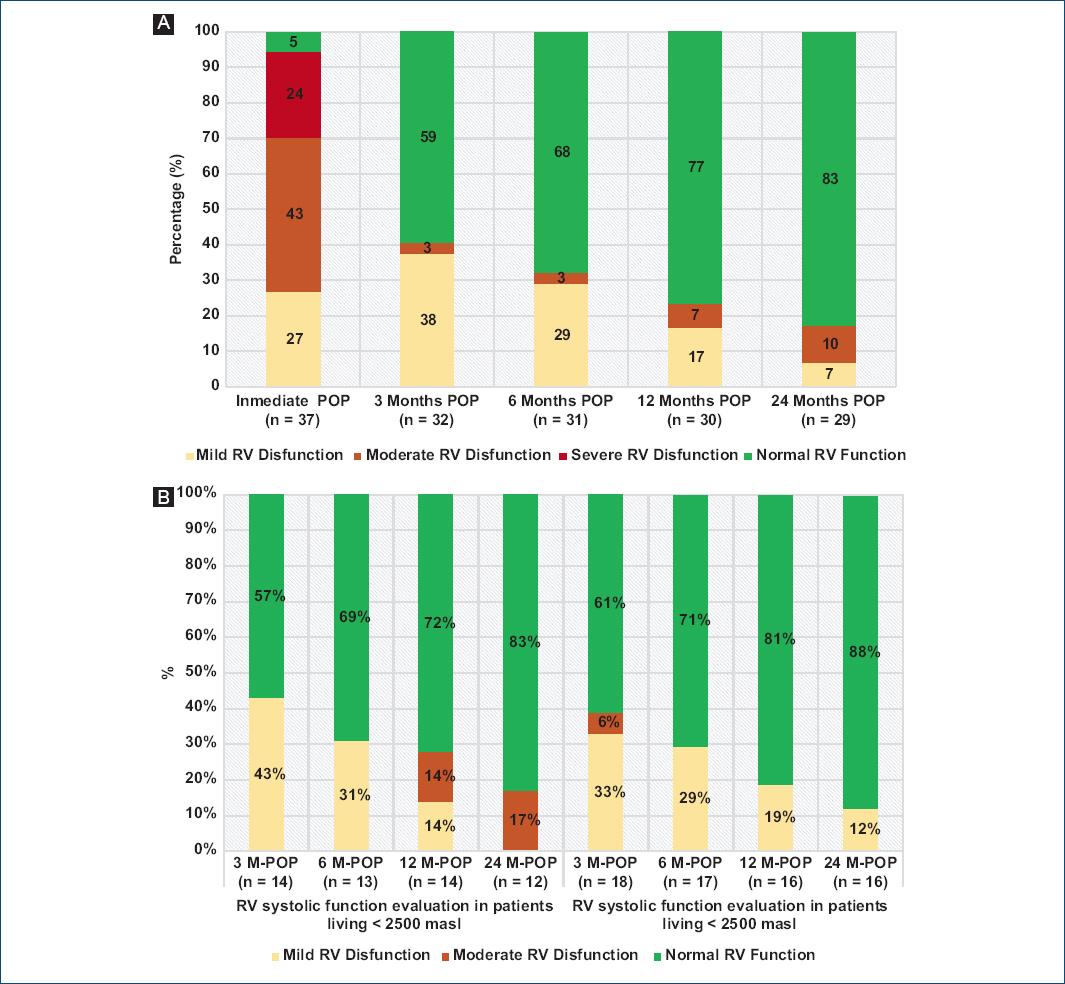
Figure 1 A: RV systolic function evaluation in different POP-HTx periods. B: RV systolic function evaluation in different POP-HTx periods in patients living at more than 2,500 masl and less than 2,500 masl. M-POP: months POP; RV: right ventricle.
The mean TAPSE in the immediate postoperative period was 8.9 ± 4.9 mm. There was a significant improvement at the 3rd month post-transplant (15.1 ± 3.6 mm), which was maintained during follow-up. At the 24th month TAPSE approached to a normal value (15.8 ± 4.9 mm) (Fig. 2A).
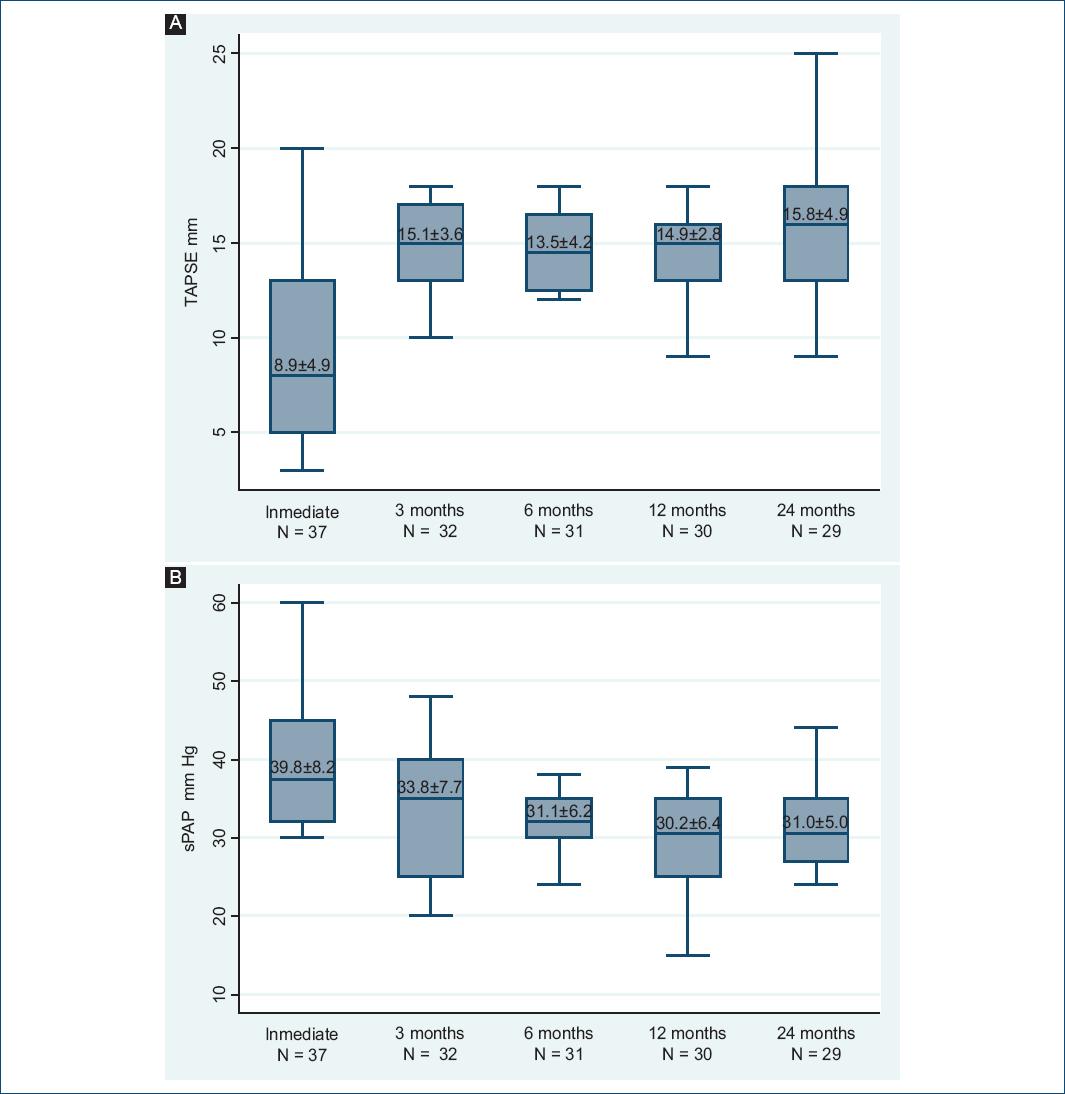
Figure 2 A: tricuspid annular plane systolic excursion (TAPSE) evaluation. B: systolic pulmonary artery pressure (sPAP) evaluation.
Some degree of tricuspid regurgitation (TR) was present in 81% of HTx-patients in the immediate postoperative period. The highest sPAP value was registered in the immediate postoperative period (39.2 ± 8.2 mmHg), with a 33% decrease in the 6th month (31.1 ± 6.2 mmHg), having then a stable behavior during follow-up (Fig. 2B).
Abnormal inferior vena cava dilation was identified in 29.7% of the patients in the immediate post-operative period. At the 3rd month post-transplant, abnormal dilation was present in only 6.2% of patients and was then normal, during the follow-up (Table 4).
A total of 24 TTE were available for the second evaluation of patients transplanted between January 2018 and May 2019. All patients lived at more than 2500 masl before and after HTx. The results of the second evaluation showed a high concordance with respect to the following registered variables: sPAP (r = 0.98), TR-severity (r = 0.87), and TAPSE (r = 0.69) (Table 5).
Table 5 TTE parameters correlation between historical data and a second observer in HTx patients in 2018-2019
| Characteristic | Historical record | Observer 2 | Differences test | Interobserver concordance (r) |
|---|---|---|---|---|
| TAPSE | 14.5 ± 4.6 | 14.7 ± 4.1 | 0.78 | 0.69 |
| sPAP | 40.2 ± 11.6 | 39.6 ± 12.9 | 0 | 0.98 |
| Tricuspid insufficiency | 34.8 | 34.8 | 0.75 | 0,87 |
| Normal, n. (%) | 39.1 | 43.5 | 0.88 | |
| Mild, n. (%) | 13 | 4.3 | 0.22 | |
| Moderate, n. (%) | 13 | 17.4 | 0.01 | |
| Severe, n. (%) | - | - | 0.07 |
sPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion; n: number, %: percentage.
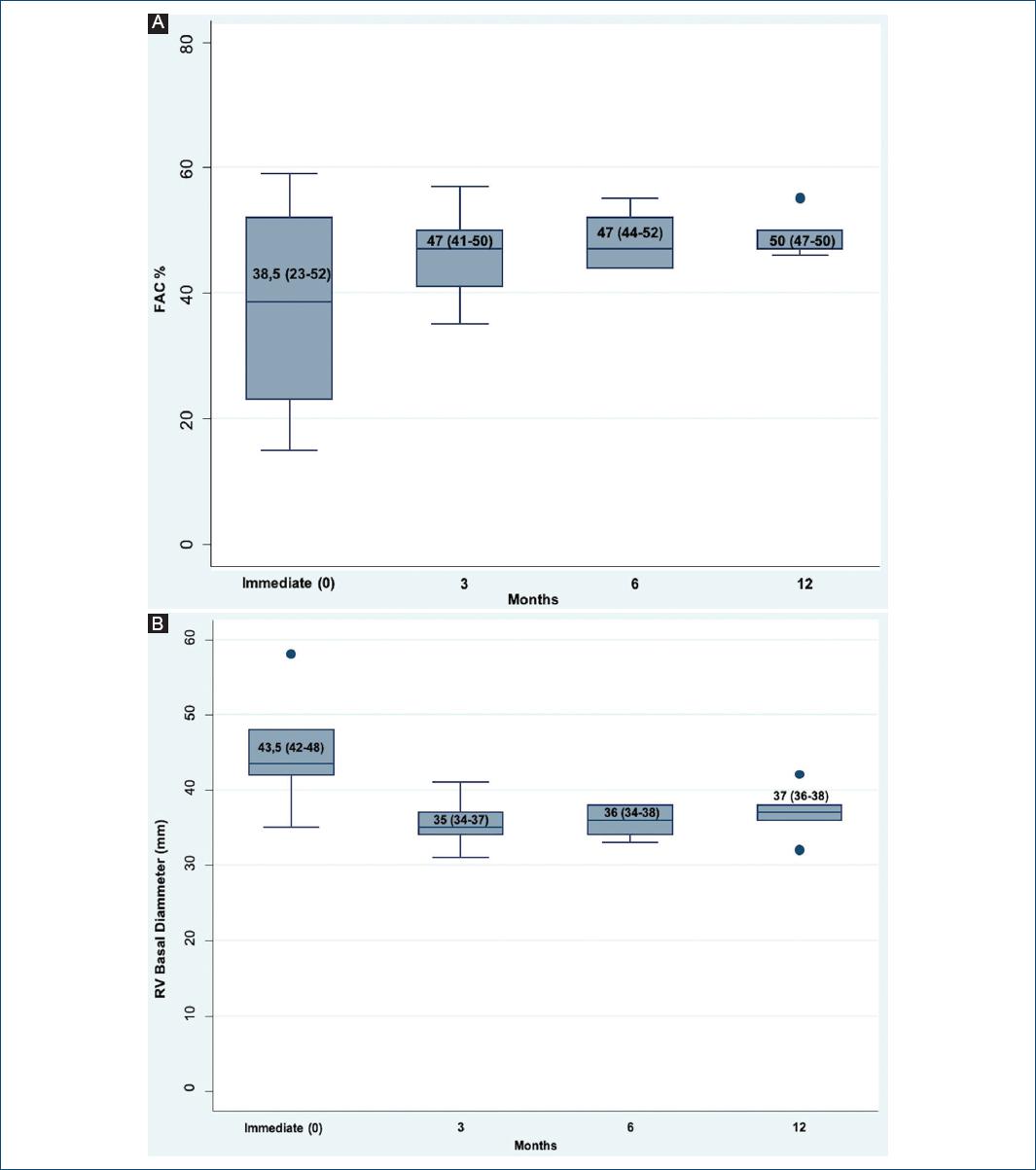
A measurement of the RVFAC and the basal diameter of the RV was performed. The results showed RV dysfunction and dilation in the immediate POP period: FAC: 38.5% (23-52%) and RV basal diameter: 43.5 mm (42-48), respectively. However, all patients presented recovery of these parameters at the 3rd month after POP, with a FAC of 47% (41-50%) and a basal diameter of 35mm (34-37), remaining so until the 1st year of follow-up (Figs. 3A and B). LVEF of transplanted patients was normal throughout the follow-up (Table 4).
Discussion
A total of 91 patients underwent HTx in our hospital, during the past 14 years. Echocardiographic results were available in 37 patients (40.6%), 95% presented RV dysfunction in the immediate postoperative period, which was predominantly moderate (43%). After the 3rd post-operative month, improvement in RV function was observed. Patients with moderate disfunction decrease from 43% to 3%. These data are similar to the one reported by Mastouri et al.6 in a study carried out in Indianapolis (A city located at 218 masl) where RV dysfunction was observed in 100% of transplants in the immediate POP period, 41% with severe dysfunction and 59% with moderate dysfunction. These results suggest a high incidence of RV dysfunction in the immediate POP period of HTx patients, possibly due to sudden increase in the RV afterload, secondary to residual pulmonary receptor hypertension, significant TR, and time prolonged ischemia7. There are no published data on the relationship with the recipient’s residence altitude.
At 12th and 24th months, two thirds of our patients (77-83%) were classified as normal RV function. The rest of the patients were classified as having mild dysfunction, and only 7%-10% were classified as having moderate dysfunction. This trend in improvement was greater than reported in the Indianapolis study, where after the first POP year, only 55% of patients showed improvement in RV function6.
Other studies have reported lower incidence of RV dysfunction with values close to 30%8, suggesting influence of additional factors not considered in this study, such as immediate POP care protocols, surgical technique, ischemia time, monitoring and care in the medium and long term, donor’s, and recipient’s pulmonary pressure, among others.
Taking in account that there is some RV dysfunction in the immediate POP, guidelines for assessment of post-HTx patients recommend the use of the 6th month POP-ETT as a reference, expecting a peak function recovery in the 1st year POP7, as we documented in our study.
A few years ago, cardiologists made a qualitative estimate of the size of the RV, comparing it with the left ventricle9. The evaluation of RV systolic function was also qualitative (“eye-ball” estimation)10, with only a few quantitative parameters available (TAPSE and tissue Doppler velocity of the tricuspid annulus), as in our study. However, RV quantification guidelines are now available5. At present, a multiparametric strategy is recommended for the classification of RV dysfunction11.
RVFAC and 3D echocardiography volume and function evaluation have a good correlation with cardiac magnetic resonance imaging (cMRI). However, methods such as 3D evaluation are not yet widely available. When possible, cMRI should be the method of choice as it is considered the “gold standard” for size and function ventricles evaluation.
Studies comparing subjective RV evaluation by TTE against cMRI have shown moderate correlation in volume estimation and low correlation in systolic function10. RV quantitative values obtained by 2D ETT compared with cMRI in patients undergoing HTx showed FAC as the value with the best correlation (r = 0.747, p < 0.001). FAC value ≤ 48.5% has a sensitivity and specificity of 90.5% to predict a reduce RV ejection fraction (< 50%) estimated by cMRI (AUC: 0.96; 95% CI, 0.908-1.013)12. This RVFAC cutoff point is higher than the previously of 35%, described as normal in the ASE guidelines5. We should keep in mind that populations are different, and the value of 48.5% specifically refers to post-HTx patients. A small group of patients with PH analyzed by López-Candales et al.13, established a cutoff point of 40.9% as a normal value. Larger trials with different population groups are needed to establish better “normal” values.
In the patients in whom the RVFAC was measured in the second evaluation (transplanted patients between 2018 and 2019), the immediate POP values showed RV dysfunction according to the cutoff point defined by the ASE (< 35%)5. However, these values showed an improvement from the 3rd month, with levels close to those established in the literature, as normal, for patients with HTx (40-48%)12,13. These results showed the same behavior as other qualitative and quantitative parameters found in the medical records (Fig. 3A)
Another quantitative parameter available in most of our population was TAPSE and its behavior showed a similar trend to the qualitative evaluation of RV systolic function. TAPSE decreased markedly, but increased from the 3rd month of POP, and remained that way at follow-up. Values almost reach the normal range after month 24 (Table 4 and Fig. 2A). The non-return to normality in the quantitative parameters of RV evaluation has been previously described in the literature6,7.
PHT after HTx is a risk factor that increases postoperative mortality14,15. In our study, the majority of patients showed a progressive decrease in sPAP. After 24 months, none patients had PHT. The 33% reduction in sPAP estimated by TTE in the first 6 months was similar to that described in a cohort from Coimbra, Portugal (Located at 499 masl), in which sPAP decreased from 46 mmHg to 31 mmHg16.
Regardless PHT etiology, it was expected that living at more than >2,500 masl could have a deleterious effect; however, it seems that it does not. At altitudes > 2,500 masl, the oxygen partial pressure is decreased by 20%, which cause a constrictive pulmonary vascular response that guarantee blood flow to a better ventilated alveolus. In addition, there is a 20-30% decrease in cardiac output compared to cardiac output at sea level. These changes occur within hours after exposure to altitude and in chronic exposures can lead to adaptive changes3. Patients with pulmonary comorbidity (Chronic obstructive pulmonary disease) expose to moderate altitudes (1780 masl) have an increase in mPAP values compared to those living at lower altitudes. However, this apparent overload does not seem to affect systolic function and performance of RV which suggests adaptation processes17.
In our cohort, 30% of the patients returned to cities located < 2500 m above sea level, 3 months after HTx. Comparative analysis of RV hemodynamic behavior and sPAP does not seem to produce significant differences. However, because the follow-up time is highly variable among study subjects and it is a retrospective study, it is not possible to make comparisons that allow evaluating the effect of confounding variables.
Data from HTx in patients living at more than 2500 masl have not been published. To the best of our knowledge, Bogota-Colombia is the world’s highest city where HTx is currently performed.
General characteristics of the population described in this study specially, recipient age, donor age, body mass index, kidney function, and 5-year survival, were similar to data published by ISHLT for 201918. Heart failure etiology was mainly non-ischemic cardiomyopathy, mostly idiopathic dilated cardiomyopathy and chagasic cardiomyopathy, the latter because Colombia is an endemic area for Chagas disease. In general, distribution pattern by etiology was similar to the one that has been described worldwide18.
Comorbidities were identified in < 25% of the population, which can explain lower complication rates.
In the recipient evaluation, our population had slightly higher pulmonary vascular resistances than average values described by ISHLT in adult population18. This could be related to altitude. Some studies have shown that at higher altitudes there is a slight increase in mean pulmonary artery pressure and in pulmonary vascular resistance19,20.
Regarding the PRA I and II, the ISHLT reported a positive value (> 10%) in 13.4%-22% of patients (both, PRA I and II) between 2001 and 201818. In our cohort, positive values (> 10%) were present in 18% of patients (PRAI: 16% and PRAII: 18%). PRA positivity have impact on early or more severe organ rejection incidence, especially when the positivity level of these antibodies is > 10% with an impact in survival reduction at values > 25%21.
Adequate gender and weight concordance are important to prevent rejection and major complications. The previous studies have shown that gender incompatibility (donor woman/recipient man) increases rejection risk in more than 3 times22. According to ISHLT gender mismatch was presented in 16.1% of HTx patients between 2010 and 201818. In our cohort, gender mismatch occurred in 11% of patients, being less than worldwide reports.
Weight mismatch, defined as a donor weight < 70% of recipient’s weight, has also been associated with mortality increased in short and long term. This association is lost with obese donors (BMI ≥ 30)23. In the present study, weight mismatch was not documented.
Limitations
The present study has limitations that must be taken into account.
First, its retrospective nature significantly limited information access, with more than 30% of data loss during follow-up. The data were conditioned to electronic files access and some non-digital records. The latter was more difficult to achieve, favoring information biases. It should be noted that similar studies describing RV behavior in HTx patients in different clinical momentums are scarce, and with sample sizes of 20-50 patients. Multicenter prospective studies will be necessary to overcome these limitations. However, due to the low rate of cardiac transplantation per year in our institution, prospective data collection may take several years and will probably require the collaboration of the other institutions that carry-out HTx in Bogotá. Taking into account the premise that this is the only city in the world that performs this type of procedure at more than 2500 masl; to date, this study is the only one that reports results in HTx patients residing at that altitude.
Second, difficulties in estimating RV dysfunction incidence in Post-HTx patients include lack of criteria standardization, which is favored by a different behavior of transplanted heart compared to non-transplanted ones and the methods used. In our study, TTE analysis and categorization were mainly qualitative, with scarce RV quantitative parameters. There were also difficulties doing TTE re-evaluation due to inaccessibility of studies carried out before 2018. With the second analysis of TTEs available from 2018, on which we obtained an adequate correlation between the observers, we tried to support the results obtained from analysis of the TTE reports taken from the clinical records. In addition, the RVFAC values (To date one of the most precise parameters of RV evaluation by TTE, after 3D assessment) were adequately correlated with the other function variables described, supporting the results, and conclusions presented. It would be interesting to carry out this same analysis in a prospective study using FAC, 3D RV assessment, and cMRI evaluation. Prospective studies evaluating RV behavior in different HTx-POP momentums using FAC by ETT have not been performed.
Finally, an adequate analysis of early graft dysfunction or rejection and its relationship with altitude was not possible due to limited data available.
Conclusions
HTx population in our center is similar to patients transplanted in different centers around the world. This may correspond to a uniformity in criteria and treatment applied in these patients.
After HTx, the majority of patients presented moderate to severe right ventricular dysfunction probably due to the recipient’s PH, which improved rapidly in the 3rd month after HTx.
Despite high altitude physiological changes in cardiovascular and pulmonary systems, HTx at 2640 masl and subsequent stay of patients at this altitude seems to have no significant impact on sPAP and RV systolic function evaluated by TTE in the immediate POP and in medium and long-term follow-up. This is probably related to a fast-onset adaptation phenomenon, adequate patient selection, and optimal post-transplant treatment.
Key points
To date, there are no data published on the performance of the RV and pulmonary pressures in transplant patients at more than 2,500 masl.
The previous studies established physiological changes in the pulmonary vasculature from 1,800 masl with an increase in pulmonary artery pressures; however, this does not affect the efficiency of transplanted hearts at more than 2,500 masl.











 nueva página del texto (beta)
nueva página del texto (beta)