Introduction
Heart transplantation (HT) continues to be the treatment of choice for patients with advanced heart failure. However, despite advances in the management, acute graft rejection is common during the 1st year after transplantation. Patients remain asymptomatic until they have a marked hemodynamic compromise that has generated an interest in finding a method that allows early identification to provide early management. Acute rejection alters ventricular repolarization, thus prolonging the QT interval, so electrocardiographic follow-up can be a useful tool in identifying this population. We present a case where this electrocardiographic abnormality allowed the identification of this condition.
Case presentation
A 31-year-old male started 1 day before admission with pain in the upper right limb, dizziness, nausea, diaphoresis, and paleness followed by loss of postural tone and loss of consciousness 3 min long. He denied previous similar episodes, fever, diarrhea, chest pain, or head trauma. The patient's vital signs, neurological and cardiovascular examinations on admission were normal. The patient history was notable for HT because of dilated cardiomyopathy 6 years before the current even. Immunosuppressive therapies at the time of presentation included cyclosporine, mycophenolate, and prednisolone without recent changes. The patient denied taking herbal supplements. A differential cell count, leukocytes, hemoglobin, blood glucose, and electrolyte levels were normal, with cyclosporine levels in subtherapeutic range (122.8 ng/mL). An electrocardiogram (ECG) showed sinus rhythm, with a pattern of right bundle branch block, with a QT interval of 420 ms and a QTc interval by Bazzet's formula of 530 ms. R-R distance was 0.64 s (Fig. 1A) that had not been documented on his follow-up ECG in the outpatient heart transplant consultation. His transthoracic echocardiogram (TTE) documented severe left ventricular dysfunction (left ventricle ejection fraction [LVEF]: 20%), with generalized hypokinesia, right ventricle of normal size but with systolic dysfunction (tricuspid annular plane systolic excursion [TAPSE] 10 mm and peak systolic velocity of tricuspid annulus color tissue Doppler 4 cm/s) and global longitudinal strain (GLS) was −5.5%. The right heart catheterization document increased pressures (central venous pressure 18 mmHg, wedge 27 mmHg, and mean pulmonary arterial pressure of 30 mmHg) and coronary angiography without vasculopathy. Endomyocardial biopsy (EMB) (Fig. 2) revealed acute humoral and cellular rejection (Grade 3R ISHLT). The specific donor antibody was positive against HLA Class II DRB *1.
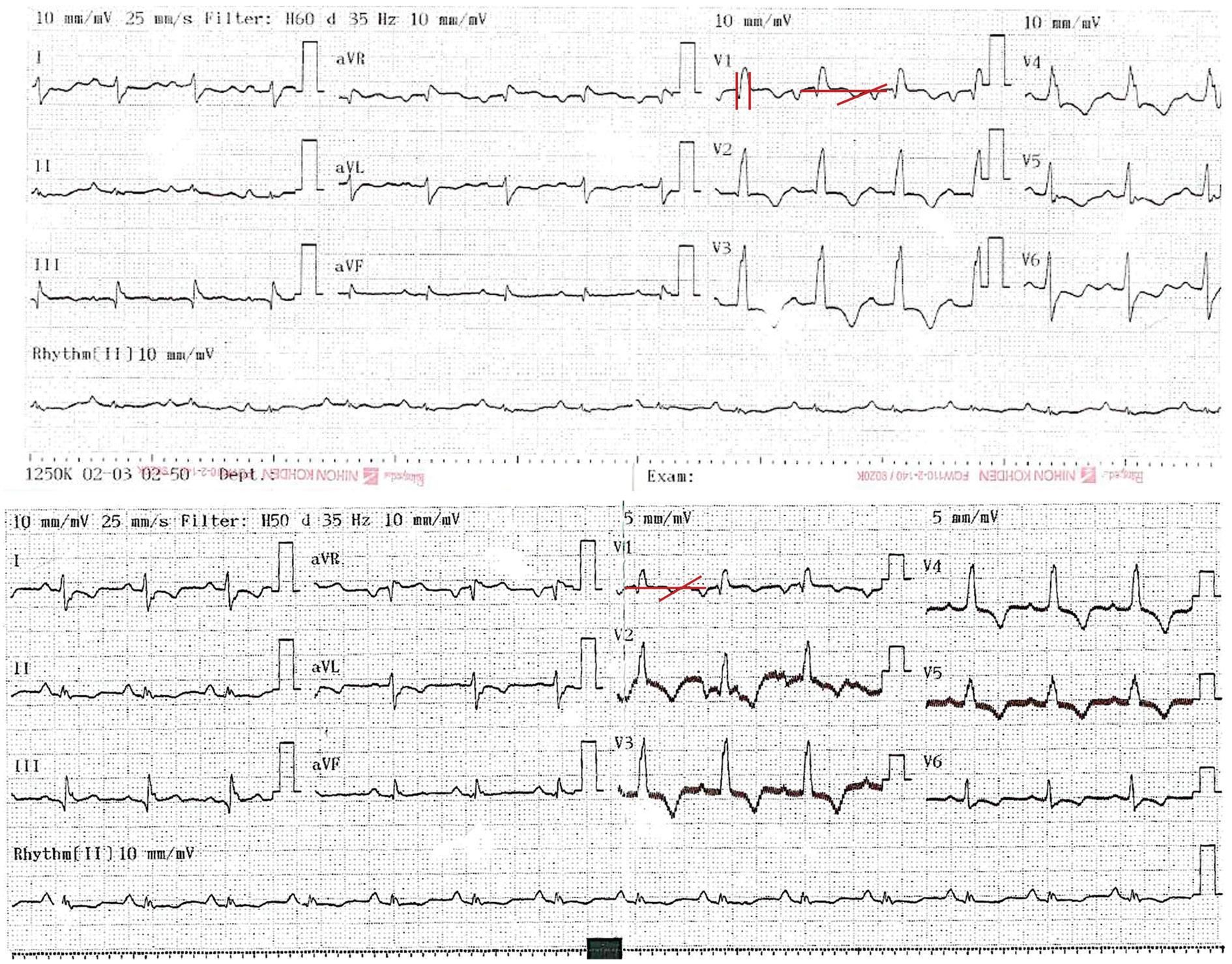
Figure 1 A: electrocardiogram on admission to the emergency: sinus rhythm, QRS of 100 ms with the right bundle branch block morphology. QRS axis slightly deviated to the left. QTc interval by Bazett's formula 530 ms and QT interval of 420 ms. Heart rate: 96 bpm, R-R distance: 0.64 s. B: electrocardiogram after anti-rejection management: QTc of 424 mseg by Bazett's formula and QT interval of 360 mseg, Heart rate: 83 bpm. R-R distance: 0.72 seg.
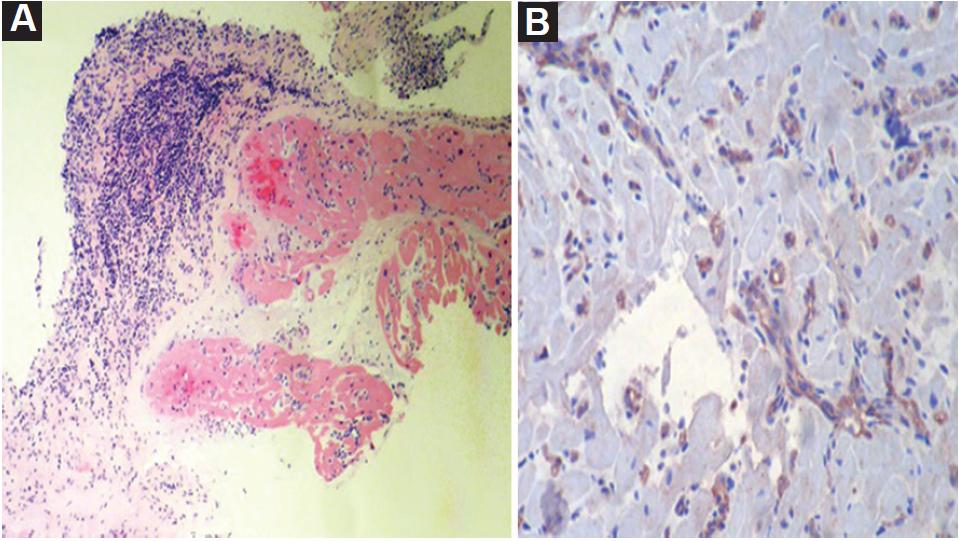
Figure 2 Endomyocardial biopsy: A. Extensive interstitial and perivascular lymphocytic infiltrate, with several foci of myocytic damage. Some capillaries with leukocyte marginalization. B. Immunohistochemistry for C4d: focal positivity in approximately 60% of capillaries: Grade 3R ISHLT.
With these findings consistent with an acute rejection of the graft, steroid bolus was initiated as well as consolidation therapy with plasmapheresis for 2 weeks followed by immunoglobulin and subsequent rituximab. The electrocardiographic changes were corrected after completion of plasmapheresis and immunoglobulin management (Fig. 1B; QTc of 424 mseg by Bazett's formula and QT interval of 360 mseg, heart rate: 83 bpm), with resolution of symptoms. His TTE showed a LVEF 55% without segmental contractility disorders, with recovery of the right ventricular function (TAPSE 21 mm, S´-wave velocity 10 cm/s) and GLS of −6.6%. The control EMB showed focal lymphocytic and 30% CD4 positivity in capillaries (Grade 1R ISHLT), concluding improvement with the management to a mild rejection. He was discharged home with an immunosuppression management adjustment as the cause of rejection was considered to be related to subtherapeutic levels of cyclosporine.
Discussion
HT is the treatment of choice for patients with advanced heart failure and provides 90% survival in the 1st year after surgery1. However, despite advances in the management of immunosuppression, acute graft rejection is common during the 1st year after transplantation with an incidence of 40%, generating 12% of deaths in this period, which makes it the leading cause of death1. These rejection events have been associated with an increased risk of death and are an independent risk factor for graft vasculopathy1. The symptoms are usually very subtle or non-existent in the early phases of these complications. It is not until the patients show overt heart failure due to hemodynamic compromise that clinical data become apparent. Although EMB is the gold standard for the diagnosis of acute graft rejection, IT is an invasive procedure that is not without risk with reported complication rates between 0.5 and 1.5%2. Several investigators reported that electrophysiological changes of the transplanted heart may be used to diagnose rejection. Wunk-Wjonaretal3 reported that the electrophysiological H-V interval was prolonged in acute allograft rejection. Kitamura et al.4 showed that the effective refractory period of the conduction system and the ventricle was prolonged during rejection episodes. However, the measurement of the repolarization duration is thought to be limited in spontaneously beating hearts because of variations of the heart rate. Therefore, we chose the QT interval and corrected it for heart rate (QTc) to normalize the measurement to a heart rate of 60 beats/min5. In a recent retrospective study, Auer et al.6 assessed the repolarization phase of paced epimyocardial electrograms. They reported a sensitivity of 84% and a specificity of 81%. However, the major disadvantage of this method is the necessity of an implanted telemetric pacemaker and special computer analysis. Hence, an attempt has been made to find a simple, non-invasive marker to identify the early stages of acute rejection. The ECG is a widely available and inexpensive tool whose findings may be useful since in patients with graft rejection, ventricular repolarization is altered, increasing the action potential, which at the electrocardiographic level will manifest as a prolonged QT interval7. This electrocardiographic alteration has also been associated with mortality8. The NEW HEART study included 151 men and 69 women transplanted with 969 biopsies/ECGs: there were 280 mild rejection events and 12 episodes classified as moderate/severe rejections. The increase in the QRS duration, as well as the duration of the QT, QTc, and PR intervals associated with moderate-severe rejections9. Richartz et al.7 found that prolongation of the QTc interval has a sensitivity of 86% and a specificity of 88% for the diagnosis of graft rejection. On the other hand, the histological grade has been strongly correlated with the increase in the QTc interval, and in general, the electrocardiographic changes precede the histological signs of rejection with their resolution or shortening of the QTc interval when there is an effective treatment of the event. QTc interval normalization also happens before the histologic resolution of the rejection7,8, as occurred in our patient.
The histologic degree of rejection correlated strongly with the increasing prolongation of the QTc interval, although the relationship might not be causal. Richartz et al.7 showed that QTc interval prolongation precedes histologic signs of rejection. After successful antirejection treatment, the QTc shortens earlier than the histologic signs of rejection (cellular infiltration) disappear. This phenomenon might be related to cell edema and concomitant myocyte electrical alterations.
As could be noted in the patient and the studies that have been published, QT interval surveillance has a key role in identifying patients with higher immunological risk and in recognition early in patients with acute rejection. These QT interval changes generally appear earlier than histological alterations.











 text new page (beta)
text new page (beta)


