Surgical correction of congenital heart diseases (CHDs) that involve the right ventricle outflow tract (RVOT) favors anatomical and functional alterations of the pulmonary artery valve and trunk in the late post-operative period. For the correction of these alterations, the patient must undergo new surgical events that require implantation of biological devices such as allografts and bioprostheses, among others, which usually deteriorate as a consequence of an adaptive patient response that modifies their geometry, favors caliber reduction and blood flow reduction, which clinically is observed as functional class deterioration, with significantly high morbidity and mortality rate being associated with these reoperations1-4.
In the late 20th century, it was thought that pulmonary valve insufficiency, one of the usually described CHD surgical repair complications that involve RVOT, would be well tolerated by the right ventricle (RV); however, experiences observed in recent years have shown that an important number of patients evolve to right heart failure, lethal arrhythmias, and sudden death5-9. Current criteria after RVOT repair in patients with complex CHD, such as tetralogy of Fallot, include clinical criteria (functional class, signs of heart failure) and RV functional variables (ejection fraction, end-systolic [ESV], and end-diastolic volume [ESV])9.
Since most patients with CHD that involves RVOT will require reoperation during their lifetime, the need for options to improve approaching strategies and decrease primary correction reoperation complications was a reason for the development of new technologies aiming to restore the blood flow to the pulmonary circulation, with percutaneous technology appearing for the implantation of pulmonary bioprostheses1-9.
In 2000, Dr. Bonhoeffer performed percutaneous implantation of a pulmonary valve in a 12-year-old patient with significant RVOT obstruction for the 1st time; this fact was the basis for the development of the Melody® percutaneous valve10. Two years after the first percutaneous implantation, the first series of eight patients with percutaneous valve successful implantation in pulmonary position was published11, and in the next few years, the learning curve and technology evolution allowed to demonstrate the safety and efficacy of this approach, mainly a less invasive method, with less bleeding risk and less infectious complications in comparison with the surgical approach11,12.
The Medtronic Melody® valve (Medtronic Inc., Minneapolis, MN, USA) was approved by the Food and Drug Administration in 2010 as part of the alternatives for the treatment of LVOT dysfunction13. Since then, its use has spread quickly, and today, more than 10,000 percutaneous valve implantations with this method are reported in the world14. The Melody® valve consists of a 34-mm platinum-iridium bare stent (CP stent, NuMED Inc.; Hopkinton, New York, USA), with one valve made with a bovine jugular vein segment. The device is attached to a balloon catheter and released on a 22-Fr sleeve1-10. Despite its size, the use of the Melody® valve was intended for those patients with RVOT diameters of < 24 mm, which limited the benefit to a specific group of patients. Thus, the need for options for RVOT of larger diameter led to the development of Edwards Sapien® valve (Edwards Lifesciences INC., Irvine, CA, USA), thus expanding the possibility of a percutaneous approach to RVOTs with a diameter larger than 29 mm, mainly in prosthetic conduits; and in specific situations, in selected patients with native tracts15.
Although the Melody® valve is the most widely used device for the percutaneous implant, worldwide studies show similar results in terms of its medium-term results, despite the fact that the first post-implant follow-up series indicate higher residual gradients with the use of the Sapien® device16. Current criteria for prosthetic valves percutaneous implantation in the RVOT are divided according to the presence or absence of symptoms and include:
In asymptomatic patients: (a) RV significant dilation: EDV > 150 mL/m2 BSA or ESV > 80 ml/m2 BSA; (b) RV systolic dysfunction, RVEF < 45%; (c) sustained atrial/ventricular tachycardia; (d) RVOT obstruction, RV systolic pressure (RVSP) > 75% of systemic pressure or higher than 80 mmHg; and (e) significant progressive tricuspid regurgitation.
In symptomatic patients: (a) moderate to severe respiratory failure and (b) significant stenosis: RVSP > 60 mmHg3-5,8-11,17.
One of the principles for the decision of this alternative for RVOT dysfunction is to precisely understand the anatomy. Analysis of each case is necessary and involves imaging studies, heart rate evaluation, hemodynamic evaluation, stress tests, and complementation of the function with the use of cardiac magnetic resonance imaging (CMRI) and cardiac computed tomography (CCT)11-13,18. Furthermore, there are different factors to determine the possibility of percutaneous implantation, with the most interesting being: (a) RVOT morphology, (b) RVOT distensibility and compliance, and (c) coronary anatomy. Up to five RVOT morphology types have been described; the prototype (due to its frequency) is the pyramidal morphology, where the base is wider with progressive narrowing toward its most distal part, which is also the most complex for placement. When this technique started being used, patients with native tracts were practically discarded as percutaneous treatment candidates; however, with the advent of techniques such as pre-stent, the Panorama was improved for these cases in which implementation of this technique became possible18-20.
RVOT distensibility and compliance can be characterized with techniques such as CCT and CMRI, which allow assessing the degree of obstruction, calcification, and pulsatility. Coronary anatomy is another essential factor, given the relationship with RVOT and increased risk of coronary occlusion during implantation5,17-20. Current standard indications for prosthetic valve percutaneous implantation include RVOT dysfunction in patients with prosthetic conduits, as well as dysfunction of surgically placed valves using the procedure known as valve-in-valve; and current case series even suggest the safety and efficacy of the use of these devices in native tracts with the alternative of techniques such as pre-stent, which in addition reduces the risk of complications such as fractures and the need for reioperation19,20.
The use of the percutaneous procedure involves risks and complications, starting with the technique during its placement: coronary occlusion, ischemia, and perforation. Post-placement complications include, among the most common: stent fracture, paravalvular leaks, endocarditis, thrombosis, and valve stenosis. Infective endocarditis has been identified as a significant complication after implantation, with the reported incidence being 2.5-4%/patient-year17. No specific characteristics have been determined regarding its microbiology, although there are series that report lower frequency17,21.
Initial experience has identified stent fracture as the complication with the highest potential, which increases the risk of stenosis, insufficiency, or embolization; in this context, stent fracture is the most common cause of reoperation, with its cause being attributed to a significant increase in stress by volume overload, most times without stent integrity loss (type 1) and without implying clinical significance. The factors with the highest association are young patients (including pediatric patients), high RVOT gradients, and small RVOT diameters, with the incidence of this complication being lower than 20% of cases22.
These percutaneous-implantation devices have shown in the USA and in European countries very low morbidity and practically no mortality and have become a real tool for patients with these complications, who also have elevated surgical risk1,10-21.
Considering the elevated cost of this technology, its arrival to Mexico has been limited; however, development in training at the pediatric interventionism department of the 20 de Noviembre National Medical Center (CMN Centro Médico Nacional) has promoted its acquisition, with good results in its implantation for correction of anatomical anomalies of patients undergoing a primary surgical correction, who present with long-term deterioration. We present the experience with Melody® valve percutaneous implantation in four cases attended to at the Pediatric Cardiology Department of ISSSTE CMN 20 de Noviembre.
From January 2016 to December 2017, four patients (three females) with an average age of 19.2 years and weight of 52.7 kg underwent Melody® valve percutaneous implantation. The predominant diagnosis was tetralogy of Fallot (75%). Two of the patients showed pulmonary insufficiency progressive increase on echocardiographic follow-up and two showed significant RVOT stenosis. Two patients had prosthetic conduits in ventriculopulmonary continuity and two required previous pulmonary valve surgical replacement. All four patients underwent pre-stenting, in different variants (see description in each case), as well as coronary circulation evaluation. The characteristics are described in Table 1 and Fig. 1.
Table 1 Demographic characteristics of patients undergoing Melody® percutaneous valve placement in the pulmonary position
| Age (Years) | Gender | Weight (kg) | Underlying diagnosis | Previous surgery | |
|---|---|---|---|---|---|
| 1 | 17 | Female | 61.5 | Tetralogy of Fallot | Lung correction + replacement with Nº 19 Edwards® prosthetic valve |
| 2 | 14 | Female | 36 | Truncus arteriosus type 1 | Rastelli procedure with Nº 16 Contegra conduit® |
| 3 | 25 | Female | 52 | Tetralogy of Fallot | Rastelli procedure with Nº 16 Contegra conduit® |
| 4 | 21 | Male | 61.5 | Tetralogy of Fallot | Lung correction + replacement with Nº 19 Edwards® prosthetic valve |
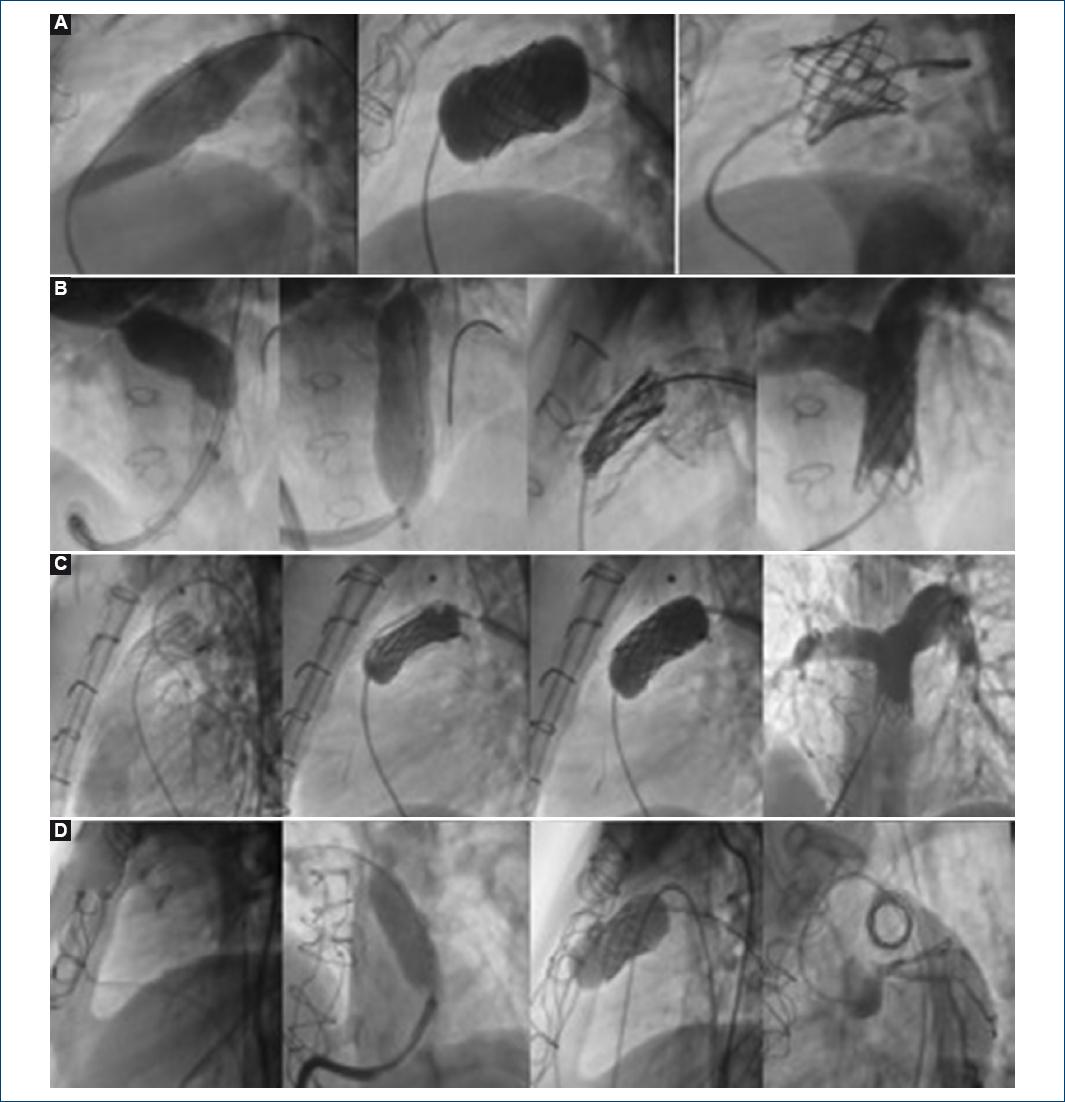
Figure 1 A: Case 1. Edwards® prosthetic valve rupture sequence with an Atlas® balloon. B: Case 2. pulmonary branches sequential plasty. C: Case 3. Melody® valve pre-stenting and opening on the balloon. D: case 4. Melody® valve placement on prosthetic ring.
Clinical cases
Patient 1
Seventeen-year-old female patient, who underwent tetralogy of Fallot correction at 4 years of age and pulmonary valve replacement with No. 19 Edwards® prosthetic valve. During her follow-up, double prosthetic valve injury was detected: with stenosis and progressive severe insufficiency (8-mm vena contracta), in addition to functional class deterioration. Before percutaneous valve implantation, RVOT pre-stenting and prosthetic ring rupture with 20 × 2 mm Atlas® balloon were performed. The final peak-to-peak gradient was 4 mmHg.
Patient 2
Fourteen-year-old female patient, truncus arteriosus type 1 carrier, repaired at 30 days of life with Barbero-Marcial surgical procedure; at 10 years of age, she required surgical reoperation with Rastelli surgery and Contegra® prosthetic conduit implantation. During her follow-up, bilateral stenosis of branches was detected; therefore, by percutaneous intervention, she had 12 × 29-mm Atrium Advanta® V12 stents placed in both pulmonary branches with kissing balloon technique. In a subsequent follow-up, and according to her weight increase, she required lung tree sequential rehabilitation with stent sequential plasty in pulmonary branches with Atlas® balloons, with right branch final diameter of 19.39 mm and left branch 19.79 mm being obtained; critical stenosis in the prosthetic conduit due to calcification (25 mmHg peak-to-peak gradient) was also discovered. Melody® valve was successfully implanted.
Six months post-implantation, echocardiogram revealed Melody® prosthesis decreased internal lumen of up to 12.6 mm, with recoil being appreciated; valvuloplasty was then decided using a 20 × 40 Atlas balloon, with adequate dilation being achieved at the recoil site with 5-mmHg residual gradient and subsequent angiography with no evidence of respiratory failure.
Patient 3
Twenty-five-year-old female patient, diagnosed with tetralogy of Fallot that required surgical correction at 5 years of age, with Rastelli procedure and No 16 Contegra® prosthetic conduit placement. She attended our institute in 2009, where echocardiogram revealed critical prosthetic conduit stenosis, and functional class deterioration was clinically determined. She underwent 40 × 14 mm uncovered conduit Palmaz® stent placement and sequential stent plasty with No. 18 and 20 Maxi LD balloons. During diagnostic catheterization, RVSP 2/3 above systemic pressure was detected, with percutaneous valve implantation, therefore, being successfully performed.
Patient 4
Twenty-one-year-old male patient diagnosed with tetralogy of Fallot with surgical correction at 5 years. During follow-up, he developed progressive pulmonary insufficiency; at 17 years of age, he underwent lung replacement with 19-mm Edwards® prosthetic valve. During his 5-year subsequent follow-up, double injury was documented: stenosis and severe insufficiency (8-mm vena contracta). He underwent Melody® successful implantation.
The characteristics before and after the implantation are detailed in Table 2.
Table 2 Characteristics before and after valve implantation
| Primary Indication | Preplacement parameters | Post-placement parameters | Pre-stenting | Complication | |
|---|---|---|---|---|---|
| 1 | Insufficiency | *VC: 8 mm †Gr: 20 mmHg | *VC: 1mm †Gr: 4 mmHg | Prosthetic ring rupture | No |
| 2 | Stenosis | *VC: 6 mm †Gr: 25 mmHg | *VC: 1mm †Gr: 5 mmHg | Palmaz® stent | Recoil |
| 3 | Stenosis | †RV Pressure: 60/0/8 †LV Pressure: 100/0/55 | †RV Pressure: 30/0/5 †LV Pressure: 110/0/60 | Palmaz® stent + sequential plasty | No |
| 4 | Insufficiency | *VC: 8 mm | *VC: 1.1 mm | Palmaz® stent | No |
Gr: gradient; RV: right ventricle; LV: left ventricle.
*Vena contracta (VC), measured by echocardiography (Phillips Epiq® 7 Ultrasound).
†Peak/peak gradients and pressures were measured by cardiac catheterization.
Discussion
We describe our initial experience of percutaneous valvular prosthesis implantation in a small cohort of patients with RVOT obstruction with good results.
Pulmonary valve percutaneous implantation is acquiring a key role in improving the failure of pulmonary bioprosthetic valves and/or dysfunctional conduits placed in the RVOT to the pulmonary artery. The two devices currently available (Melody® valve and Edwards Sapien® valve)13-16 have international reports in the treatment of this group of patients, but not in our setting. On the other hand, the selection of eligible patients for pulmonary valve replacement represents a challenge for the success of the procedure, and thus generating an exchange of cases would help decrease this risk18-22. Consequently, one of the main challenges for the future is to expand percutaneous pulmonary valve implantation to a larger patient population. Hence, we consider important sharing the experience of those centers that are currently working with this type of technology, with the purpose to improve patient outcomes, expand pulmonary valve percutaneous implantation therapy, and continue reducing the number of reoperations in this population.











 nova página do texto(beta)
nova página do texto(beta)


