Introduction
Worldwide, 5.4 million new cases of cardiovascular diseases are diagnosed, most of them related to coronary artery atherosclerosis, and cause more than half a million deaths annually and an economic expenditure of billions of dollars1. The outlook in Mexico is not different, and ischemic heart disease is estimated to have 1% annual incidence in patients aged 45-54 years and 4% in individuals aged 75-84 years2; in addition, it is the leading cause of morbidity and mortality.
The role of coronary stents in the treatment of ischemic heart disease, both in its acute and chronic behavior, is now well established, and it is currently the most common modality for the performance of coronary artery bypass graft3,4.
The application of coronary interventionism as therapeutic behavior for ischemic heart disease has been limited in this country. According to the Organization for Economic Cooperation and Development (OECD), Mexico ranked last in the use of this resource among the 35 OECD member countries in 20135; furthermore, the results of the national registries of acute coronary ischemic syndrome (RENASICA Registros Nacionales del Síndrome Isquémico Agudo) I, II, and III6-8, which have included 20,647 patients, have shown that the use of this therapeutic resource only occurs in 29% of them, which is attributed to the high cost of the required supplies (stents, fluoroscopy room, etc.), among other factors, and the health expenditure deriving from the use of this therapeutics is high and thus this need cannot be adequately covered.
Mexico has a considerable lag in science and technology investment, calculated at approximately 0.44% of the gross domestic product (GDP); in the USA, expenditure in relation to the GDP is 2.79%. This is reflected on lack of development and technological dependency, and thus, there is an urgent need to improve this gap that actually accentuates inequality9.
Materials and Methods
Phase I: Desired characteristics and initial design
The design and development of the INC-1 coronary stent started in late 2014 at the National Institute of Cardiology, in association with GSE Biomedical and the Monterrey Technology and Higher Education Institute, with the support of CONACYT. To that end, a multidisciplinary group was formed, weekly meetings were punctually arranged (a total of 12), where specific tasks and technical requirements were resolved, with methodology being used as a guidance based on quality function deployment (QFD)10; with this tool, the needs of the customer, in this case interventional cardiologists, were confronted against technical or engineering requirements and limitations and were compared with the maximum objective to be achieved, after taking into account, the biophysical characteristics of state-of-the-art commercial stents. Among the requirements requested by the customers or interventional cardiologists, the following stood out: low mechanical fatigue, little or no corrosion, no ionic release, magnetism or favorable hydrophobicity, zero galvanic effect, flexibility and maneuverability, low profile, sufficient radial force, minimal longitudinal shortening, uniform radial and longitudinal expansion, easiness for mounting, appropriate cell area for drug release, minimal surface area, high biocompatibility, and minimal cell proliferation.
The characteristics the INC stent should have were agreed: L605 cobalt and chrome (Co-Cr) platform, in accordance with the international standard [(Standard Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS R30075)], with 80 μm thick struts, balloon expanded and with equal or higher performance than second-generation stents, depending on the requirements of standard ISO 25539, vascular stents11.
CAD drawings of the different design iterations were carried out using the SolidWorks program, in addition to multiple tests, until the most suitable model for the requested needs and requirements was defined before manufacturing; finally, a balance of the most favorable values with regard to radial force, flexibility, and longitudinal deformation, among others, was found.
Phase II: Prototypes and physical tests in vitro
Thirty stents were manufactured in an FDA-certified laboratory by laser cutting on an 80 μm thickness Co-Cr L605 tube, with the final design specifications (Fig. 1); passivation and electropolish treatment were carried out. With 15 pieces, in vitro tests were carried out in accordance with the ISO standard (evidence folder is available)11:
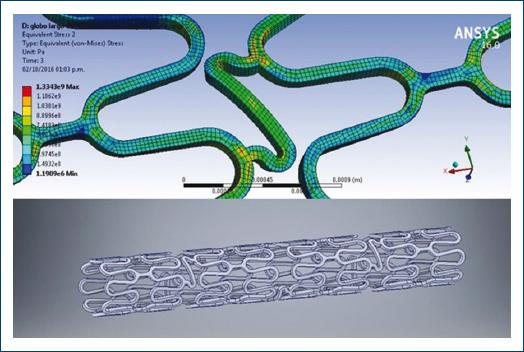
Figure 1 The image on top shows the finite element structural analysis (numerical model for structural analysis of the coronary stent deformation plasticity). The image below shows the stent final structural model.
1.Post-expansion length change.
2.Longitudinal diametrical uniformity.
3.Uniformity of stent struts expansion, interconnecting beams behavior, in comparison with those obtained in the computer simulation.
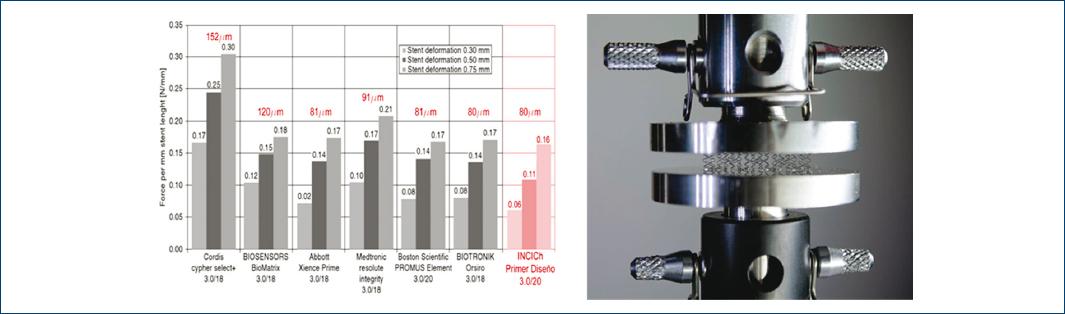
4.Diametric compression test between parallel plates or radial force and comparison with different commercial stents according to ISO 25539-2 standard11 (Fig. 2).
5.Longitudinal compression test.
6.Migration test.
Phase III: Start of the experience on animal phase
At this initial stage, a preliminary test of the stent was carried out in two animal models (one in rabbit and one canine) to assess safety and efficacy; a protocol was subsequently conducted in pigs with clinical, angiographic, and intracoronary ultrasound follow-up of 4-6 months, with adherence to the guidelines established by the institutional bioethics committee and international standards for the use and care of experimental animals12-15.
Based on the above, the INC coronary stent was placed on a New Zealand breed rabbit model of 4.4 kg in the abdominal aorta and in a 22 kg canine model of the Nahuatl breed in the right renal artery. The purpose was to assess technical success regarding the following biomechanical properties: navigation capacity, maneuverability, symmetrical expansion of the stent, adequate fixation to the vascular wall, no compromise of secondary branches, and ease of balloon removal; in addition, safety of the latter was assessed with regard to vascular integrity, blood flow quality, thrombosis, and involvement of secondary branches. The recommendations of the preclinical stent evaluation consensus were taken into account16.
Technical procedure
The procedure was carried out at the experimental surgery laboratory of the animal care facilities of the National Institute of Cardiology in an operating room that has a portable fluoroscopy equipment (Siemens Arcadis Varic, model 08677051), with care for asepsis, antisepsis, and balanced general anesthesia (propofol and isoflurane), through a percutaneous femoral approach in both models with a 4 Fr introducer for the rabbit and 6 Fr for the dog; angiograms were obtained to define the stent diameters; then, the biomechanical properties of the stent were assessed to determine its technical and safety success, and a 3.0 × 20 mm INC stent was implanted in the rabbits abdominal aorta and one measuring 3.5 × 20 mm in the dogs right renal artery. A control angiography was obtained, in addition to intravascular ultrasound (Boston OptiCross tube Scientific), from the canine model renal artery stent and, in both, clinical follow-up for 3 weeks was established.
Results
Efficient and guided processing was achieved using the QFD of INC-1 stent desired foundations. Regarding the results for mechanical behavior (radial force, flexibility, deformation to elongation, and longitudinal shortening, among others), the stent showed characteristics that were highly adequate and consistent with the virtual computational tests evaluated using finite element. (The full results of computational and mechanical tests, under the guidelines of the ISO 25539-2 standard, were the deliverables of a GSE project supported by CONACYTs innovation stimulus program, whose evidence folder is available).
In both models (rabbit and canine), by means of the fluoroscopy-guided implant, satisfactory technical success was demonstrated in all assessed parameters, among them an adequate capacity of the releasing system to reach the implant site by percutaneous approach, and achievement of adequate maneuverability and acceptable fluoroscopic visualization, with efficacious and accurate release at the implant site, which confirmed symmetrical expansion and complete fixation to the vascular wall. In addition, a satisfactory result was obtained in terms of safety, since in both models was the treated vascular anatomy respected, with its integrity being preserved, without extravasation, with normal final angiographic flow, and no evidence of thrombi (Fig. 3A).
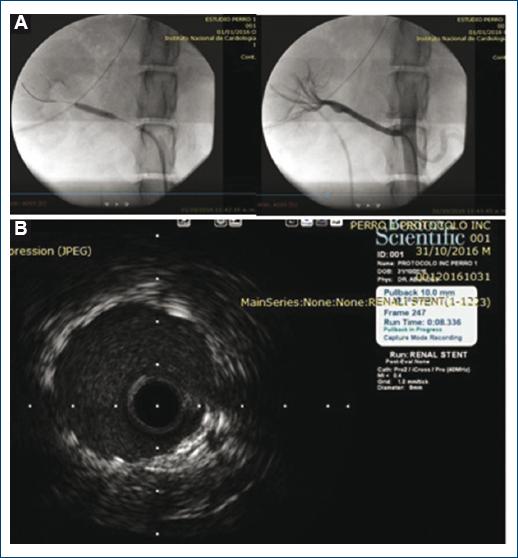
Figure 3 A: Fluoroscopic image of stent release over the balloon in the dogs right renal artery and final angiographic image with an optimal result. B: Ultrasound image with adequate apposition, fixation, and expansion results.
Intravascular ultrasound (OptiCross probe, Boston Scientific) applied to the canine model renal artery stent, corroborated adequate expansion and fixation of the stent to the vascular wall, symmetry in three analyzed segments, and consistent diameters achieved with the selected balloon diameter of 3.5 mm (Fig. 3B).
Discussion
Coronary artery interventionism with stent placement has been shown to be a safe and efficacious procedure in the treatment of ischemic heart disease due to coronary artery atherosclerosis, which is the most common cause of worldwide disability and mortality17. This project has been devised with the purpose of having a national technology development that promotes not only the obtainment of a more accessible product for the Mexican population but also innovation and less dependence on foreign technology.
The objectives of the National Institute of Cardiology are based on the principles of assistance, education, research, and technological development, outlined since its foundation by doctor Ignacio Chávez. Based on these needs, the Sub-directorate of Innovation and Technological Development has been created in the institute.
There are precedents of stent development in Mexico18-20; however, technological improvement has been highly important in recent years, and thus, currently, there are recommendations and requirements for design and computational, mechanical, and preclinical tests that ensure the best clinical results16-21.
In accordance with the above, carrying out this project to design, manufacture, and test, a coronary stent with all the modern recommendations is fully justified; in a second stage, efforts will be merged with institutions or companies to develop this project for industrial and commercial production, in such a way that patients, the institute, Mexican companies and the country are benefited, both for the decrease of technological dependence from abroad and for the creation of new jobs and development of qualified human capital. Hence, a virtuous circle will be created based on this program, and the coronary stent will, therefore, be the first of other technology projects to be developed in the area of cardiovascular health.
An accurate and detailed description of engineering processes (design, finite element testing, etc.) was not included for the purposes of this publication, only the most important aspects of this area for medical knowledge; such results were provided in detail to CONACYT (there is a folder of evidence available), which justified the engineering procedures used for this project.
Conclusion
This document described how the INC-1 stent was conceived and manufactured, the first tests of which were carried out satisfactorily. The purpose was to develop technological resources of our own at the National Institute of Cardiology, to benefit a growing population of patients with ischemic heart disease. That is why a metallic coronary high-tech stent has been developed, with quality material and certification for medical use and a novel cell design. The next step is development of a drug-eluting stent.
It is essential for this device to be tested in a sufficient number of experimental animals, whose model can be comparable to the human being to obtain clinical and statistical validity and guarantee its safe use; later on, developing a protocol in human subjects according to international ethical and scientific standards will be possible22.











 nova página do texto(beta)
nova página do texto(beta)