Introduction
Paravalvular leak (PVL) is a relatively common and important complication, which occurs in 5-17% of surgical valve prostheses1-3 as a consequence of surgical suture failure favored by the presence of calcium, infection, tissue friability, or non-circular shape of the ring4,5.
Although most PVL are small and asymptomatic, 2-5%4,6,7 have clinical relevance and can cause heart failure and hemolytic anemia and are associated with poor clinical results1,3,8,9.
Surgical re-intervention for closure of the defect or prosthetic replacement has been the classical treatment. It has shown less mortality in comparison with PVL conservative treatment8,10, but it is accompanied by high perioperative morbidity and mortality, as well as an elevated rate of residual or recurrent leaks, especially in the presence of multiple previous operations and comorbidities. In addition, in clinical practice, some patients cannot undergo the intervention due to increased surgical risk, which implies a poor prognosis4,11-15.
Transcatheter closure is an emerging therapeutic alternative that has been shown to reduce the severity of the leak and its symptoms, with variable success rates and a low rate of complications. PVLs localization, their morphology, number, size, trajectory, as well as access, technique, and devices used have been described as factors that can influence on the results6,14,16-18. PVL have variable anatomical characteristics, which makes it very difficult for a device to fit in all cases. The specific selection of the device is based on the size, shape, and localization of the leak to completely occlude the defect without interfering with valvular function. Multiple devices that are not specifically designed for this purpose have been used, including the devices of the Amplatzer group (Abbott), which are currently the most widely used: Amplatzer Vascular Plug III (AVP III) in Europe and AVP II in USA19-22.
The purpose of this study is to analyze the feasibility and immediate and 6-month results of post-operative PLVs transcatheter closure with the use of occlusion devices in the authors institution.
Methods
The registry of a consecutive series of patients with surgical prosthetic PVLs that were closed through transcatheter with occluder devices at Ignacio Chávez National Institute of Cardiology from Mexico, between January 2006 and December 2016, was used.
PVL was defined as the presence of jet regurgitation originating between the edge of the prosthetic ring and the surrounding native tissue observed by Doppler echocardiography. Regurgitation severity was defined according to the parameters established by the American Society of Echocardiography23-25. The transcatheter closure technique, route of access, type of implanted device, and the use or not of echocardiography during the procedure were decided at the operators criteria. In general, for the election of the size of the device, the larger and smaller diameters of the defect were taken into account, as determined by two-dimensional or three-dimensional (3D) transesophageal echocardiography, and a device sized ≥ 1-2 mm than the reference diameters was used.
For all cases, baseline characteristics, echocardiogram findings, procedural details, hospital outcomes, and follow-up were collected from electronic medical records. All patients remained on clinical follow-up for at least 6 months, during which they underwent an echocardiographic control study. The follow-up results regarding the degree of residual leakage, change in functional class, vascular complications, device embolization, death, myocardial infarction, need for valve operation after device implantation, hemolysis, reoperation, stroke, infective endocarditis, and re-hospitalization were searched. After completing the procedure, technical success was defined as device implantation at the level of the PVL without interference with normal operation of the prosthesis or need for urgent intervention, and procedural success when, in addition to technical success, PVL reduction ≥ 1° was achieved. Functional class according to the New York Heart Association (NYHA) classification was assessed at baseline and at 6 months. Clinical success at follow-up was considered when clinical improvement ≥ 1° in functional class was found within the following 180 days after the closure procedure. Hemolytic anemia was defined by clinical documentation of symptoms together with laboratory tests for anemia and hemolysis16.
Results
During the analyzed period, 21 valvular prostheses were operated (15 mitral, 5 aortic, and 1 tricuspid) during 20 procedures. In one patient, two leaks were closed at different localizations (mitral and tricuspid) in a single procedure. Of the cases, 58% corresponded to males, average age was 45 ± 18 years and 50% of cases had two or more previous sternotomies. Average time from valve operation to PVL closure was 9.4 years. Demographic and clinical baseline characteristics are summarized in table 1.
Table 1 Patient baseline clinical characteristics
| Demographic and clinical characteristics | n = 20 |
| Average age | 45 (±18) |
| Males | 11 (58%) |
| PVL prosthetic location | |
| ─ Mitral | 15 (71%) |
| ─ Aortic | 5 (24%) |
| ─ Tricuspid | 1 (5%) |
| Mechanical prosthesis | 17 (81%) |
| History | |
| ─ Atrial fibrillation | 7 (35%) |
| ─ Ischemic heart disease | 4 (20%) |
| ─ Hypertension | 4 (20%) |
| ─ Endocarditis | 2 (10%) |
| ─ Diabetes mellitus | 1 (5%) |
| ─ Previous CVD | 1 (5%) |
| ─ CKD (GFR < 60 mL/min) | 0 (0%) |
| ─ COPD | 0 (0%) |
| Pulmonary hypertension (PASP ≥ 40 mmHg) | 12 (60%) |
| Average time from valve operation to intervention | 9.4 Years |
| One prosthetic valve carrier | 16 (80%) |
| ≥ 2 previous sternotomies | 10 (50%) |
| Baseline functional class, NYHA ≥ II | 14 (70%) |
| Indication for the procedure | |
| ─ Heart failure | 10 (50%) |
| ─ Hemolytic anemia | 2 (10%) |
| ─ HF+ HA | 3 (15%) |
| ─ Echocardiographic finding | 5 (25%) |
PVL: paravalvular leak; CVD: cerebrovascular disease; CKD: chronic kidney disease, determined by a calculated creatinine clearance rate ≤ 60 mL/min; COPD: chronic obstructive pulmonary disease; PASP: pulmonary artery systolic pressure, calculated by echocardiography; NYHA: New York Heart Association classification; HF: heart failure; HA: hemolytic anemia.
The most common indications for the procedure were heart failure or hemolytic anemia in 75% of cases, and most of them (70%) were in NYHA functional Classes II to IV. In 10 patients (50%), multiple leaks were described (in 91% of cases they were serious) and left ventricle ejection fraction was on average 54 ± 17% (Table 2).
Table 2 Baseline echocardiographic characteristics, previous
| Number of leaks present per valve | |
| ─ One | 10 (50%) |
| ─ Multiple (≥ 2) | 10 (50%) |
| Localization of the mitral paravalvular leak (n = 17 leaks) | |
| ─ Posterior | 9 (53%) |
| ─ Anterior | 5 (29%) |
| ─ Lateral | 3 (18%) |
| Localization of the aortic paravalvular leak (n = 7 leaks) | |
| ─ Posterior | 3 (43%) |
| ─ Anterior | 2 (29%) |
| ─ Lateral | 2 (28%) |
| Localization of the tricuspid paravalvular leak (n = 1 leak) | |
| ─ Septal | 1 (100%) |
| Paravalvular leak degree before the procedure | |
| ─ Severe | 19 (91%) |
| ─ Moderate | 2 (9%) |
| LVEF (%) average | 54% ± 17 |
LVEF: left ventricle ejection fraction
Access was femoral in all individuals. As for aortic PVLs, all cases were carried out by retrograde approach, and in the case of mitral PVLs, in 87% of cases, an antegrade approach through a trans-septal puncture was used, while the remaining 13% were retrogradely approached from the left ventricle to the left atrium (Table 3).
Table 3 Procedure characteristics
| Closure technique | |
|---|---|
| Mitral leaks | n = 15 |
| ─ Anterograde | 13 (87%) |
| ─ Retrograde | 2 (13%) |
| Aortic leaks | n = 5 |
| ─ Retrograde | 5 (100%) |
| Tricuspid leak | n = 1 |
| ─ Anterograde | 1 (100%) |
| ─ Technical success | 19 (95%) |
| Type of device used (n = 25 devices) | |
| ─ Amplatzer Vascular Plug III | 13 (52%) |
| ─ Amplatzer Vascular Plug II | 4 (16%) |
| ─ ASD Amplatzer | 2 (8%) |
| ─ PDA Amplatzer | 4 (16%) |
| ─ VSD Amplatzer | 2 (8%) |
| Number of devices used for each PVL | |
| ─ 1 occluder | 15 (75%) |
| ─ 2 occluders | 5 (25%) |
| Use of echocardiography during the procedure | |
| ─ 3D TEE | 14 (70%) |
| ─ TEE | 2 (10%) |
| ─ ICE | 1 (5%) |
| ─ IC + TEE | 2 (10%) |
| ─ No, only fluoroscopy | 1 (5%) |
| Residual leak after the procedure | |
| ─ Absent/mild | 16 (80%) |
| ─ Moderate/severe | 4 (20%) |
| Average fluoroscopy time | 55 minutos |
| General anesthesia | 19 (95%) |
| Average follow-up time | 26 meses |
ASD: atrial septal defect; PDA: patent ductus arteriosus; VSD: ventricular septal defect; 3D TEE: three-dimensional transesophageal echocardiogram;
TEE: transesophageal echocardiogram; ICE: intra-cardiac echocardiogram;
PVL: paravalvular leak.
The device was (technically) successfully implanted 95% of the times, only in one case was there device embolization, and ≥ 1 degree of regurgitation reduction (procedural success) was achieved in 95% of cases. Twenty-five devices were implanted; the most widely used was AVP III® in 13 of the closed leaks (52%), followed by AVP II® in four leaks (16%) and Patent Ductus Arteriosus Amplatzer Occluder® in other four (16%); in addition, Atrial Septal Defect Amplatzer Occluders® were used in two leaks (8%) and Ventricular Septal Defect Amplatzer Occluders® in two more (8%) (Figs 1-3). In five procedures, two occluders were used due to the size and shape of the target leak or multiple leaks in the same valve. Transesophageal echocardiogram was used in 90% of procedures (70% with 3D technique), intracardiac echocardiogram in one case (5%), and the procedure was guided only by fluoroscopy in one case (5%) (Table 3).
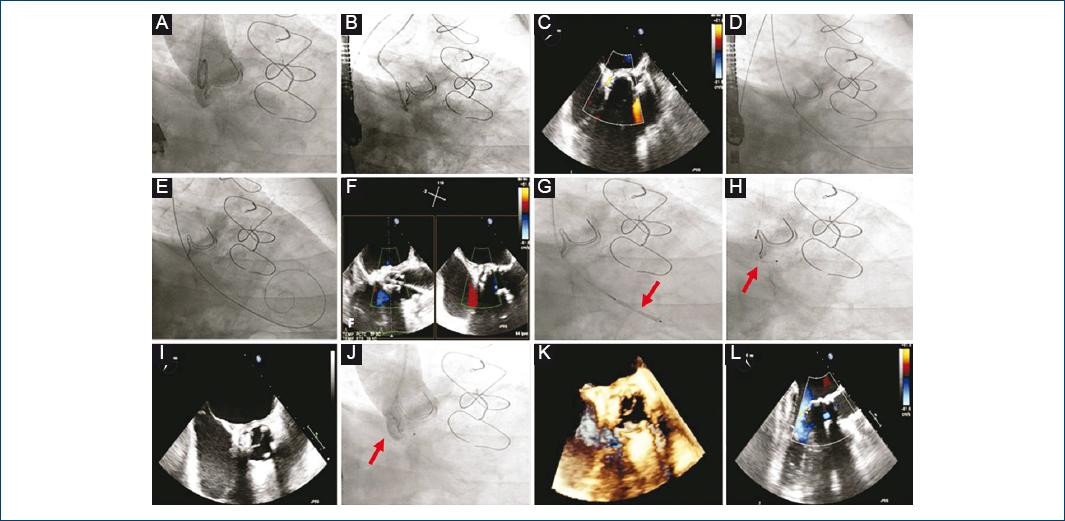
Figure 1 Transcatheter closure of an aortic paravalvular leak. Amplatzer Vascular Plug II 8 mm (red arrows). A: aortogram, severe aortic paravalvular regurgitation. B: selective leak cannulation. C: transesophageal echocardiogram (TEE), verification of the position and severity of the leak. D: retrograde crossing through the defect. E: exchange for a rigid guidewire. F: guidewire position echocardiographic verification. G: catheter advancement and occluder initial retention disk release. H: occluder final retention disc release. I: device position and valve function evaluation. J: final aortogram, slight residual regurgitation. K-L: final result with three-dimensional and two-dimensional TEE.
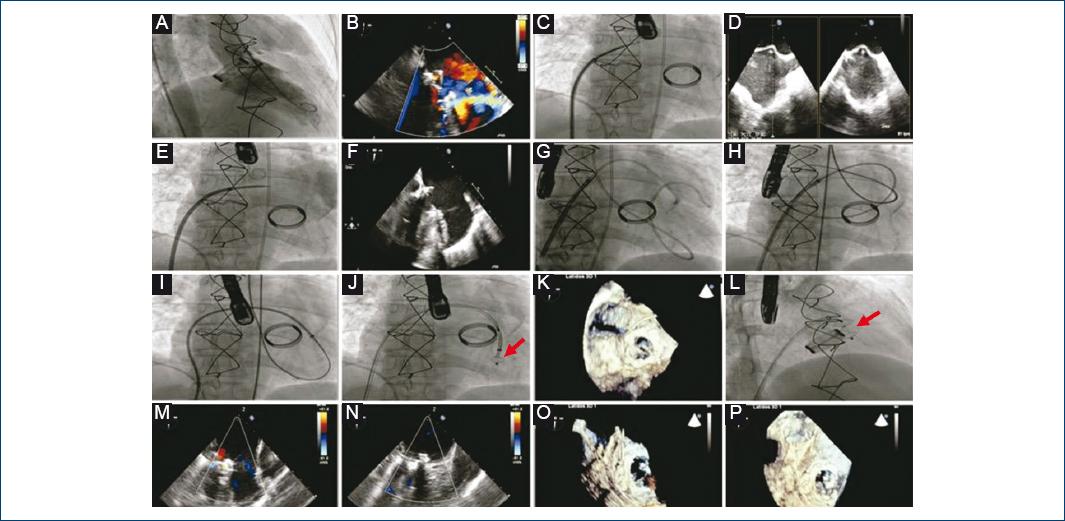
Figure 2 Transcatheter closure of a mitral paravalvular leak. Amplatzer Vascular Plug II 12/25 mm (red arrows). A: left ventriculography, severe mitral paravalvular regurgitation. B: transesophageal echocardiogram (TEE), verification of the position and severity of the leak. C: trans-septal puncture. D: TEE that guides the level of trans-septal puncture. E: angiography at the level of the left atrium. F: retrograde crossing of the guidewire from the left ventricle to the left atrium through the defect, verified by echocardiography. G-H: loop at the level of the left atrium and formation of an arteriovenous loop. I: release system anterograde advancement through the defect. J: release of occluder initial retainer disc. K: device position and valve functioning assessment. L: release of occluder final retention disc. M-P: final result by two-dimensional and three-dimensional TEE

Figure 3 Transcatheter closure of tricuspid paravalvular leak. Amplatzer Vascular Plug II 8/4 mm (red arrows). A: anterograde crossing of the defect from jugular access and advancement of the release system. B: release of occluder initial retaining disc. C: release of occluder final retention disk. D: final result by fluoroscopy.
The degree of residual leakage was zero or slight in 80% of cases and, at 6 months, 79% of clinical success was achieved. There were no vascular complications related to the procedure or cerebrovascular episodes during follow-up. At 6 months, survival was 100%; however, three cases required valve operation (15%); in one case, urgently due to embolization of the device and in the other two due to the appearance of hemolytic anemia. There were no cases of interference with prosthesis normal functioning (Table 4).
Discussion
Surgical reoperation is the regular procedure for the treatment of PVL; however, multiple series record a 30-day mortality of 6-22%. Taramasso et al. reported 98% success for the surgical procedure in 122 patients, but with a 30-day mortality of 11%, with all cases being related to cardiac causes, which suggests that it is a high-risk intervention. These results support the need for a valid therapeutic alternative to conventional operation, especially in patients with multiple previous cardiac interventions and risk factors for higher mortality (adjunct comorbidities, chronic kidney failure, and mitral PVL)26.
In the authors series, to choose transcatheter closure, in addition to surgical risk, the number of previous sternotomies (50% had more than one) and patient age (45 years on average) was considered to avoid reoperation and exposure to a larger number of future re-sternotomies, since there is a progressive increase in surgical mortality with the number of reoperations.
Technical success in the series was high, similar to that of a meta-analysis of 12 non-randomized studies, where a figure of 76.5% was published 17. This suggests that, despite being a technically complex procedure, experience of the centers, teamwork, and the use of appropriate imaging techniques allow obtaining good results in most cases.
Procedural success was also high, similar to that of the series published by Ruiz et al.6 (86%) and Sorajja et al.18 (89%), but unlike the series of the authors, the AVP III device, main occluder used in this, was not used in those, which could be related to the high success rate found for the procedure, given that its oval morphology could better adapt to the anatomy of the defect (almost always semilunar), it comes pre-assembled, has better crossing profile, and can be introduced using smaller-caliber sheaths. In five cases (25%), the use of two occluders was required, which shows that there is no ideal device for all cases and that, in large and irregular defects, multiple small devices can be better adjusted to the paravalvular space and avoid prosthetic dysfunction.
Up to 95% of the authors cases had echocardiographic guidance, since it plays a crucial role during the procedure and directs the operator during different phases of the intervention, such as choosing the right place for trans-septal puncture, appropriate crossing of the guidewire through the defect, choice of the device, and immediate evaluation of the result, with severity of the leak and its size being best estimated by 3D transesophageal echocardiography. In recent years, new technology of 3D echocardiography images fusion with fluoroscopy has been described to be able to help facilitate the success of the procedure and better assess the result27,28.
In the most recently published series, clinical success at 30 days was 72%29, similar to the authors series, where 79% of cases improved their symptoms at 6 months. There is little evidence that compares transcatheter closure versus surgical intervention. A non-randomized comparative study, published by Wells et al., reported equivalent clinical results at 1 year between transcatheter closure and surgical intervention, with significantly less postoperative intensive care unit stay, 30-day readmission, bleeding, and perioperative morbidity. Another comparative study by Angulo-Llanos et al. noted that in-hospital mortality was significantly higher in the surgical group (30.6% vs. 9.8%) and that clinical improvement was significantly higher in the transcatheter group13,29. Taramasso et al. found that transcatheter closure through transapical route in high-risk patients had lower hospital mortality in comparison with surgical closure and that 12-year overall survival was 39.8 ± 7% (although it was significantly lower in patients with more than one reoperation15).
Most patients (80%) of the authors series had no or slight residual leakage, which appears to be key to obtain a good result, since a significant relationship between the rate of cardiovascular adverse episodes, final functional class, and better long-term survival has been described30.
The procedure involves many challenges during its development and is not without complications, and constant surveillance is therefore essential. The published complication rate is low and the most common include vascular problems, urgent operation due to device embolization, interference with the prosthesis, perioperative stroke and bleeding; despite all this, death related to the procedure is uncommon6,19,30-32, which suggests that this is a safe procedure, similar to the authors findings, where the main acute complication was embolization of a device (5%). On the other hand, during follow-up, there was newly-occurring hemolytic anemia in two patients (10%), in whom atrial and ventricular septum Amplatzer occluders were used, which forced a new valve intervention, which is significantly higher than previously reported rates with only 1.6%. Consequently, the development of specific devices is necessary to overcome this important problem. In addition, end-stage heart failure has been described as the more frequent cause of these individuals death during follow-up33,34; this indicates that transcatheter leakage closure is usually practiced at an advanced stage of heart valve disease or in the presence of multiple comorbidities.
Finally, the results support transcatheter closure of PVLs as a less invasive, effective, and safe alternative, in hands of a multidisciplinary team and after detailed planning. One limitation of this work that should be mentioned is that it was based on a single registry of a small series of patients with medium-term follow-up.
Conclusions
Transcatheter closure of PVLs is a feasible, safe procedure, with high rates of technical, echocardiographic, and clinical success in the short and medium term. It is a suitable therapeutic alternative, particularly in patients at high surgical risk or with multiple associated comorbidities. Long-term comparative studies are still needed to define transcatheter closure as the first-treatment strategy for PVLs.











 nueva página del texto (beta)
nueva página del texto (beta)


