Introduction
Approximately one-third of the population in intensive care units is in a state of circulatory shock, whose rapid recognition is important to avoid tissue injury and death1.
The shock state has usually been categorized according to its cause2. Septic shock is the most severe manifestation of sepsis with an approximate mortality rate of 30%; its incidence in patients admitted to intensive care units varies from 6 to 14%3-5. The cardiogenic shock commonly described in patients with acute myocardial infarction (AMI) has an incidence of 6-9% and its frequency has remained constant during the past decades with an approximate mortality rate of 50%6.
There is little doubt about the physiopathological mechanisms of the different types of circulatory shock originally described by Weil and Shubin, however in the clinical practice at cardiac care units, it can be difficult to differentiate one mechanism from the other, which can hinder the treatment7.
This article aims to better understand the hemodynamic mechanisms responsible for the shock according to the practical approach proposed by Gonzalez et al.8
Shock mechanism
Shock is a state that compromises life, defined by a circulatory failure in which there is loss of the physiological balance between the oxygen delivery (DO2) and the oxygen uptake (VO2) conditioning an anaerobic metabolism and cellular hypoxia7. The reduction of cardiac output and/or peripheral resistances is finally translated into an increase in oxygen extraction, with the consequent decrease in central venous oxygen saturation (SvO2), which may even occur before the elevation of serum lactate. Elevation of lactate is directly proportional to the prognosis, initial values above 4.0 mmol/L and negative clearance are related to higher mortality9-11.
Circulatory shock can be classified into four subtypes according to its mechanism: (1) loss of vascular tone that causes poor distribution of blood flow (distributive shock); (2) failure of the cardiac pump function (cardiogenic shock); (3) loss of circulating volume with decreased venous return (preload) either by internal or external losses (hypovolemic shock); and (4) obstruction caused by a pulmonary embolism, tension pneumothorax, or cardiac tamponade (obstructive shock). These shock states are not mutually exclusive and can be found simultaneously. Typically, the last three states are characterized by a low cardiac output with increased peripheral vascular resistance, while in the distributive shock cardiac output is normal or high with loss of the vascular tone2,6,7.
Evaluation of circulatory shock
The diagnosis of circulatory shock is based on clinical components, hemodynamics, and biochemical data of tissue hypoxia. There are three types of clinical windows described by Vincent et al. through which we can see the effects of the altered tissue perfusion: the skin (coldness, cyanosis, and pallor), kidneys (oliguria with urinary output < 0.5 mL/kg/h), and the central nervous system (neurological alterations including drowsiness, disorientation, and confusional state). The presence of hypotension defined in the state of shock as a mean arterial pressure < 65 mmHg, systolic blood pressure < 90 mmHg or a decrease > 40 mmHg of baseline blood pressure is a component of shock2,12.
The two main biochemical markers of tissue hypoperfusion are the serum lactate and the central venous oxygen saturation (SvO2) obtained in a blood sample from the cavoatrial junction9,11,12.
The evaluation of the circulatory shock, as mentioned above, can be done in a simple way by physical examination, evaluating the windows in search of hypoperfusion data; nevertheless, an integral approach is necessary for conjunction with the biochemical variables, and hemodynamic parameters Fig. 14,13.
Hemodynamic profiles
Once the circulatory shock has been identified, it is necessary to determine the main responsible mechanism. The clinical context and the physical examination are important, but in complex situations, as it happens in cardiac care units, reaching a correct diagnosis is usually a challenge. Each shock mechanism has different hemodynamic characteristics that allow us to identify them (Table 1).
Table 1 Hemodynamic profile in different shock states
| Shock subtype | Cardiac index | Systemic vascular resistances | Central venous pressure | Pulmonary capillary wedge pressure |
|---|---|---|---|---|
| Cardiogenic LV | Low | High (Can be low in 25% of cases) | High | High |
| Cardiogénic RV | Low | High | High | Low |
| Hypovolemic | Low | High | Low | Low |
| Obstructive | ||||
| Pulmonary embolism | Low | High | High | Low |
| Tamponade | Low | High | High | High |
| Distributive | Normal/High (Can be low in the late phase of sepsis) | Low | Low | Low |
RV: right ventricular; LV: left ventricular.
Hypovolemic shock
It is characterized by a significant loss of intravascular volume resulting in an increase of sympathetic tone causing selective vasoconstriction of the skin, muscles, and splanchnic circulation to maintain venous return as well as cardiac output. If the intravascular volume loss continues, there is a decrease in the preload and subsequently in the cardiac output12,13.
Cardiogenic shock
Any cause of left or right ventricular dysfunction or both can lead to cardiogenic shock, characterized by pump failure with increased ventricular filling pressures, and a low cardiac output with increased systemic vascular resistance14.
Obstructive shock
It is caused by the inability to maintain adequate cardiac output despite normal intravascular volume and intrinsic myocardial function. An obstruction due to a pulmonary embolism, tension pneumothorax or cardiac tamponade causes a decreased cardiac output, an elevation in systemic vascular resistances and variable wedge pressure (pulmonary artery wedge pressure [PCWP]) depending on the etiology15,16.
Distributive shock
It is caused by the loss of vascular tone with the resulting maldistribution of blood flow due to sepsis, anaphylaxis, or spinal cord injuries. Usually, the cardiac output is normal or high and a normal PCWP13,15.
In 1980, Gonzalez et al. proposed a three-dimensional scheme to classify hemodynamic profiles according to three determinant variables: filling pressures, cardiac index and unlike the Forrester scale, adding the arterial pressure as a third variable with which they obtain eight possible hemodynamic states with different clinical expression and therapeutic approach, Groups 2-4 (systolic arterial pressure > 90 mmHg) correspond to patients with hemodynamic compromise but normal arterial pressure due to different compensatory mechanisms, these profiles were previously known as pre-shock, and if treated timely and properly can have a better prognosis; otherwise they will develop circulatory shock (Groups 5-8)8.
Although this classification was initially aimed to assess the hemodynamic status during AMI, currently with the availability of new monitoring devices which allow a more accurate measurement of these and other hemodynamic parameters, we consider that its adjustment may be useful to classify the different hemodynamic states observed in the cardiac care units (Fig. 2).
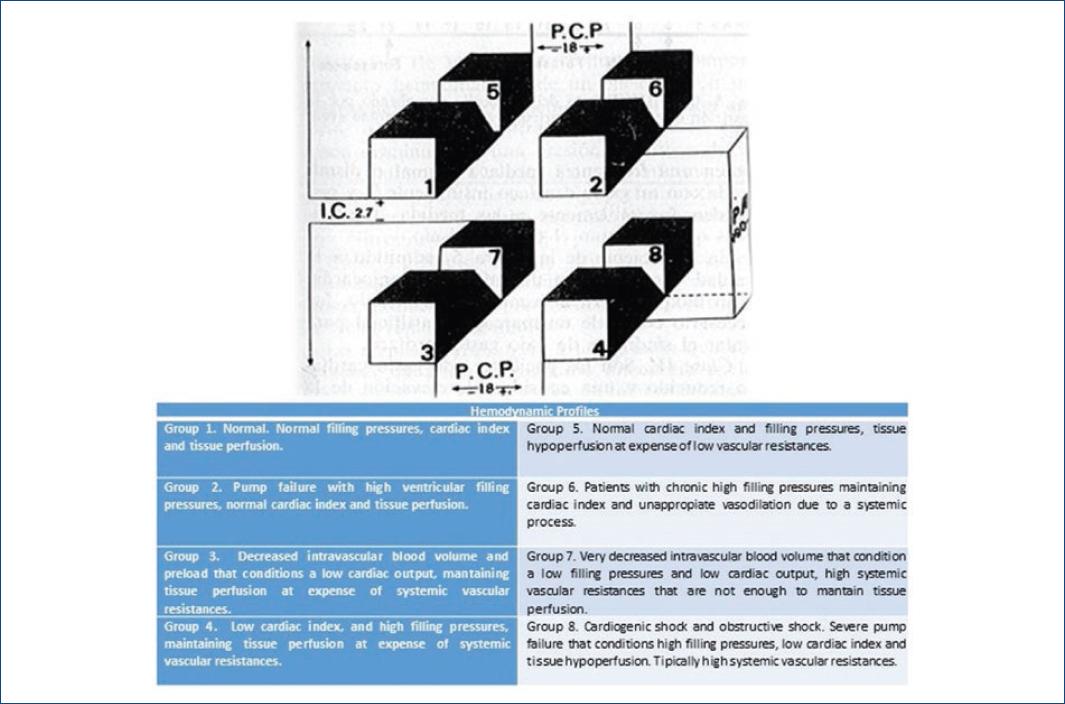
Figure 2 Hemodynamic profile in cardial care units (modified from Los problemas hemodinámicos en el infarto del miocardio. Arch Card Mex. 1980;50(3):319-26, with authorization from Dr. Jesus Antonio González Hermosillo).
An adequate initial assessment of the hemodynamic status can be achieved with the clinical examination and monitoring of certain basic hemodynamic parameters (heart rate, blood pressure, central venous pressure, respiratory variables, SvcO2, electrocardiography, lactate, and urine output). However, when this fails, there are other monitoring modalities that guide the management of fluids and the inotropic/vasopressor support (PCWP, stroke volume variation, cardiac output, extravascular water, etc.) (Table 2).
Table 2 Hemodynamic parameters
| Parameter | Equation | Normal Values |
|---|---|---|
| SaO2 (Arterial oxygen saturation) | 95-100% | |
| SvcO2 (Central venous oxygen saturation) | 70% | |
| Arterial blood pressure (TA) | Systolic diastolic | 90-140 mmHg 60-90 mmHg |
| Pulmonary artery wedge pressure (PCWP) | 5-12 mmHg | |
| Cardiac output (CO) | HR × SV/1000 | 4.0-8.0 L/min |
| Cardiac index (CI) | CO/BS | 2.2-4.0 L/min/m2 |
| Stroke volume | CO/HR × 1000 | 60-100 mL/beat |
| Systolic volume index (SVI) | CO/HR × 1000/BS | 33-47 mL/m2/beat |
| Systolic volume variation (SVV) | (maxSV − minSV)/Mean SV × 100 | 10-15% |
| Right atrium pressure (RAP) | 0-5 mmHg | |
| Systemic vascular resistances (RVS) | 80 × (MAP RAP)/CO | 800-1200 dynas/s/cm |
Hemodynamic monitoring devices
Although still the gold standard, less used, the pulmonary artery catheter was introduced in 1970 by Swan, Ganz and Forrester as a method for the measurement of cardiac output, and it is with this that several studies have compared the majority of the new devices and techniques used17.
Recently, multiple devices have been developed allowing cardiac output and other hemodynamic parameters to be obtained in real time. Among many others, these systems include PiCCO®, MostCare Vygon®, FloTrac Vigileo® Echocardiogram, and Lung Ultrasound, which provide information on preload, afterload and contractility variables, all aimed at improving both cardiac output and tissue perfusion18,19.
Non-invasive monitoring devices can be moderately invasive or minimally invasive. The moderately invasive devices (require arterial catheter plus a central venous line) offer the advantage of a continuous analysis of cardiac output by means of the thermodilution principle and minimally invasive devices (only require an arterial catheter) allow an uncalibrated analysis (FloTrac®/Vigileo®, LiDCOrapid®, ProAQT®/Pulsiflex®).
With transpulmonary thermodilution, it is possible to determine the cardiac output, extrapulmonary extravascular water, pulmonary vascular permeability, and index of cardiac function and end-diastolic volume (Table 3)20-21.
Table 3 Hemodynamic monitoring devices
| Method | Examples | Calibration | Advantages | Disadvantages |
|---|---|---|---|---|
| Transpulmonary thermodilution (moderately invasive) | PiCCO®
VolumeView® EV1000® LiDCO® |
Calibrated | Intermittent and continuous CO and other variables | Need of central venous and arterial line |
| Pulse contour and pulse pressure variation (minimally invasive). | FloTrac/Vigileo®
ProAQT® Pulsioflex® MostCare®/PRAM LiDCOrapid® |
Non-calibrated | Continuos CO | Lack accuracy in unstable patients or during use vasoactive drugs |
Pulmonary artery catheter
Catheter introduced by jugular, subclavian, or femoral access in the pulmonary artery. It allows the measurement of the PCWP, indicative of the filling pressures of the left atrium; it also allows the measurement of cardiac output by thermodilution, calculation of pulmonary and systemic vascular resistance as well as ventricular systolic volume. It is not considered a dynamic monitoring device and has wide inter-observer variability17,21.
PiCCO® system
It uses a central venous catheter and an arterial line that provides continuous measurement of cardiac output by thermodilution using a bolus of cold fluid injected through the central line. By means of an algorithm based on the analysis of the arterial pulse wave, continuous monitoring of cardiac output, and systolic volume is possible. The variation of the systolic volume and the variation of the pulse pressure are variables that can guide the response to fluid, although they are limited to completely sedated patients, under invasive mechanical ventilation and with the absence of arrhythmias. Unlike the pulmonary artery catheter, it is less invasive, allows to measure cardiac output continuously and assess the response to fluids19,22.
FloTrac/Vigileo® system
Device uses the variation of pulse pressure and vascular tone to calculate the systolic volume and cardiac output19,20,22.
Transthoracic echocardiogram
Useful to measure cardiac output by calculating the velocity-time integral of the left ventricular outflow tract by Pulsed Doppler, it is a dependent operator procedure. It is also useful to asses volume responsiveness. Table 4 summarizes the parameters that can be calculated using echocardiography23.
Table 4 Echo parameters for the assessment of circulatory shock
| Cardiac output | LVOT Area × VTI (LVOT) × HR | LVOT Area = (aortic annulus in cm)2 ×
0.785 VTI LVOT = Sample volume of the pulsed Doppler 1 cm before the valve in apical approach three or five chambers, tracing with an electronic pencil the Doppler spectrum of the aortic flow |
| Fluid responsiveness | Spontaneous breathing Invasive mechanical ventilation | IVC collapsability index > 36% or IVC < 10 mm
IVC distensibility index > 18% IVC variability 12% VTI and LVOT peak velocity variability > 12% |
| Filling pressures | Right atrium pressure Left Atrium pressure | IVC < 21 mm and > 50% collapse = 3 mmHg
IVC > 21mm and < 50% collapse = 15 mmHg IVC < 21 mm and < 50% collapse or > 21 mm and > 50% collapse = 8 mmHg E/e > 14 (High) |
| Diastolic function | Impaired relaxation Pseudonormal Restrictive |
Filling pressures − Filling pressures +/− Filling pressures + |
| Left ventricle | EF (Simpson) | Men > 52% Women > 54% |
| Right ventricle | Longitudinal function Global systolic function |
TAPSE > 17 S > 9.5 FAC > 35% |
| Lung hemodynamics | PASP mPAP PVR | TR gradient + RAP 90 − (0.62 × RVOT acceleration time) (peak TR velocity/RVOT VTI) × 10 + 0.16 |
LVOT: left ventricular outflow tract; VTI: velocity-time integral; HR: heart rate; IVC: inferior vena cava; RVOT: right ventricular outflow tract; RAP: right atrial pressure; TR: tricuspid regurgitation; PVR: pulmonary vascular resistance; mPAP: mean pulmonary artery pressure; PASP: pulmonary artery systolic pressure.
Lung ultrasound
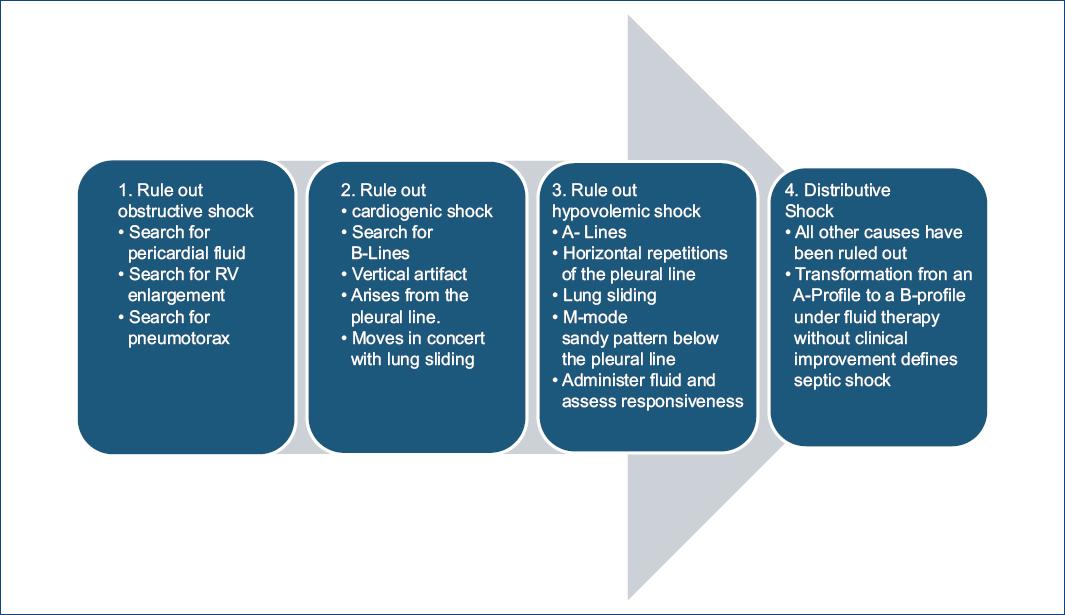
It is a tool that has been proposed for the assessment of circulatory shock using the Fluid Administration Limited by Lung Sonography-protocol first searching for pericardial fluid, right ventricle enlargement and tension pneumothorax (obstructive shock), if none of these is identified, the next step is to search for B-lines whose presence indicates pulmonary edema and cardiogenic shock as the likely cause. On the contrary, its absence, with a normal sonographic lung surface and fluid responsiveness indicate hypovolemic shock24 Fig. 3.
Goal directed therapy
The modification of all these variables (oxygen transport, preload, afterload, and vascular tone) is possible through pharmacological and non-pharmacological interventions19,20. The initial management of the shock should include ventilatory assistance, fluid resuscitation, and the use of vasoactive drugs according to the different hemodynamic profiles; occasionally, when these strategies fail and in the proper context it is necessary the use of circulatory assistance devices (Intra-aortic Balloon Pump, Extracorporeal Membrane Oxygenation, CentriMag, Impella, etc.) (Table 5).
Table 5 Pharmacologic and non-pharmacologic intervention
| Class | Tissue perfusion | CI | Filling pressures | Example | Causes | Recommendation |
|---|---|---|---|---|---|---|
| 1 | → | → | → | Normal | NA | Requirements |
| 2 | → | → | ↑ | HFpEF | Multiple | Diuretic Vasodilator NIVM |
| 3 | → | ↓ | → | Hypovolemia | Losses (GI, diuretics, bleeding, etc.) | Crystalloids Blood |
| 4 | → | ↓ | ↑ | HFrEF | Multiple | Diuretic Vasodilator NIMV STVAD LTVAD |
| 5 | ↓ | → | → | Distributive shock | Sepsis Anaphylaxis Spinal cord injury | Vasopressor |
| 6 | ↓ | → | ↑ | Valvular heart disease/HF + vasodilation | Mix | Vasopressor +/− Inotropic |
| 7 | ↓ | ↓ | →/↓ | Hypovolemic shock | Losses (Surgery, diuretics, bleeding) | Vasopressor Crystalloid Blood |
| 8 | ↓ | ↓ | ↑ | Cardiogenic shock | MI Valvular Arrythmia | Inotropic PCI IABP Pacemaker VAD ST or LT |
GI: gastrointestinal; IABP: intra-aortic balloon pump; PCI: percutaneous coronary intervention; STVAD: short-term ventricular assist devices; LTVAD: long-term ventricular assist device.
Conclusion
The importance of the different tools is to be able to provide a better and easier assessment of the different hemodynamic profiles in circulatory shock. The cardiologist must have the ability to identify and assess different hemodynamic parameters in initial stages before circulatory shock; the failure to recognize and treat coexisting etiologies and contributors to the state of shock can lead to poor prognosis.











 nueva página del texto (beta)
nueva página del texto (beta)