Introduction
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is a worldwide disease characterized by cardiovascular mortality, impaired quality of life and significant long-term complications such as recurrence, a chronic thromboembolic pulmonary disease with or without pulmonary hypertension, and post-thrombotic syndrome (PTS)1. PE – the most severe consequence – is the third cause of cardiovascular mortality after myocardial infarction and stroke, the leading preventable cause of death in hospitalized patients, the main cause of pregnancy-related maternal death in developed countries, and the second cause of mortality in cancer patients1. Furthermore, VTE is the third most common complication in trauma patients, and PE is the third most common cause of death in patients who survive the first 24 h after injury2. PE survivors commonly have persistent right ventricle dysfunction, impaired functional status (NYHA Class II–IV), diminished exercise capacity (6-min walk test), and reduced quality of life in the follow-up3. In addition, 3.8% are predicted to develop chronic thromboembolic pulmonary hypertension4.
On the other hand, up to 70% of patients with PE have DVT, and up to 32% of patients with DVT have asymptomatic PE5,6. Furthermore, PTS can be observed in up to 25-50% of DVT cases, of which, 5-10% later have severe limitations and poor quality of life. In addition, PTS exponentially increases health-care costs in the United States and Canada7. Despite this evidence, advanced therapies to reduce PTS incidence are not carried out expeditiously. Recently, Heart Teams are launched to improve the management of complex cardiovascular diseases8, including PE patients. In 2012, the Massachusetts General Hospital (MGH) created the first formal and successful multidisciplinary rapid-response team, called program evaluation and review technique (PERT), to assess and provide clinical recommendations for patients with submassive and massive PE in real time9. Worldwide institutions reproduced similar concepts, mobilizing multidisciplinary teams that coordinate and provide optimal therapeutic options, which in turn improve patient care10. However, PERTs does not include DVT – the source of PE – despite the negative impact it has in terms of quality of life and public health costs11. We designed to improve the quality of care12 of the entire clinical spectrum of VTE, the first – to the best of our knowledge – rapid response team in Mexico, called Hospital Zambrano Hellion VTE Rapid Response Team (PREVENTION-team).
Materials and methods
PREVENTION-team objectives
Primary objective: to provide fast-track stratification and diagnostics (60-90 min) after protocol activation to initiate anticoagulation alone promptly or anticoagulation plus advanced therapy (systemic or mechanical thrombolysis) in submassive, massive, and proximal DVT. The decision-making between anticoagulation alone or advanced therapy will be by an experienced clinician and depends on the extension of the thrombus burden and right ventricular dysfunction severity. Secondary objectives: (1) in-hospital increased rate of low-risk PE and distal VTE patients; (2) exploration into the cause of PE as ensuring age-specific cancer-related screening, thrombophilia testing in patients <40 years with weak triggers, thrombus in unusual sites, or strong family history10; and (3) long-term anticoagulation management: election and length of anticoagulation, adherence, bleeding complications, and management. (4) To identify those with a high-risk profile for chronic thromboembolic disease, post-PE syndrome, chronic thromboembolic pulmonary hypertension, and PTS patients10. (5) To implement a prospective registry on Research Electronic Data Capture, an online platform high-quality surveys, and databases from Vanderbilt University supported by the National Institutes of Health (https://projectredcap.org/). (6) Organize community-based support groups, and patient education to improve adherence, to reduce recurrence, and bleeding complications, with de intention of extending the concept of the thrombosis-free hospital to thrombosis-free home.
PREVENTION-team: structure and organization
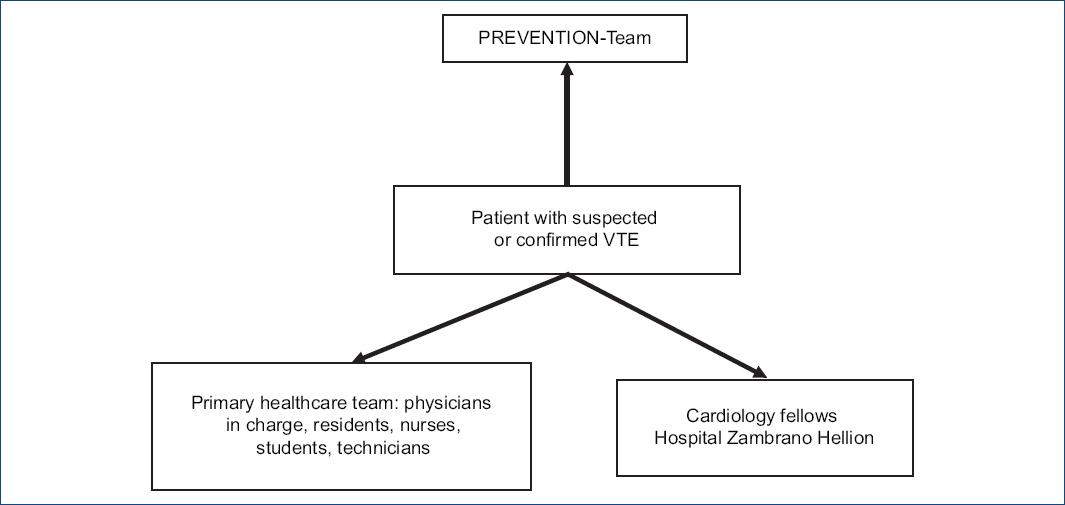
The multidisciplinary team includes physicians trained in cardiology, vascular medicine, angiology, echocardiography, cardiovascular imaging, and interventional cardiology. The team cornerstone will be the health-care team in charge (physician, nurses, residents, students, and technicians), cardiology fellows, and PREVENTION-team. Furthermore, effective coordination and communication will be mandatory for a successful program (Fig. 1). The team must be easily accessible and provide a consistent, rapid, and effective multidisciplinary response in the emergency room, intensive critical care unit, or in-hospital setting. The PREVENTION-team organization ensures a fast-track program to start specific treatment between 60 and 90 min after code activation, reproducing ST-elevation myocardial infarction, and ischemic stroke reperfusion programs.
PREVENTION-team: activation and execution
Table 1 shows the principal steps and the staff involved in the execution of the program. The first step of activation, which is based on clinical presentation (sudden dyspnea, near or syncope, chest pain such as angina, respiratory distress, and hypoxemia) suggests submassive or massive PE13 or proximal DVT (leg pain and swelling). Therefore, the hospital staff must know the VTE risk factors and how to identify high-clinically suspicious patients. Before the official launch, we will conduct educational programs, round table discussions, and case simulations geared toward hospital physicians, nurses, residents, students, and technicians. Furthermore, patient education will be mandatory to improve the outcome and reduce recurrence and bleeding complications in the follow-up.
Table 1 Key steps, events, and personnel in the execution of the PREVENTION-team protocol
| Phase | Key event (s) | Key members | |
|---|---|---|---|
| Pre-activation and activation | – VTE detection and/or suspicion by referring MD or
member of the team in charge – A call placed to PREVENTION-team line |
Referring MD Hospital residents |
Nurses Medical students |
| Initial response | On-call cardiology fellow: – Calls back referring MD – Gathers case history – Notifies PREVENTION-team members of event and plans online meeting |
On-call cardiology fellow
Referring MD |
|
| Response | – Online meeting – The case will be present by an on-call fellow with images and laboratory results – Consensus treatment will be mandatory – Treatment recommendation is given to the primary health-care team in written form |
On-call cardiology fellow
Referring MD PREVENTION-team |
|
| Transfer | – Transfer patient to necessary department (ICU, OR, and catheterization laboratory) | On-call fellow Nurses and hospital staff |
|
| Execution | – Carry out planned treatment – Immediate revascularization |
Catheterization laboratory personnel
OR personnel PREVENTION-team |
|
VTE: venous thromboembolism; ICU: intensive care unit; OR: operating room.
An activation line will be available 24 h a day, 7 days a week, and 365 days a year. The cardiology fellow on call will be responsible for the protocol activation, immediate patient evaluation, and obtain imaging and laboratory studies to accelerate the diagnostic process and save time. This information will be present during an online meeting. The checklist called S2HIELDB (S signs and symptoms, H history, I image, E Electrocardiography, L laboratory, D demographics, and B bleeding risk) provides the team with the necessary information to establish a high-clinical suspicion, diagnosis, bleeding risk, and decision-making (Table 2). Risk stratification will be base on clinical presentation, echocardiogram, and biomarkers findings. Imaging techniques and or ultrasound will prove the final diagnosis.
Table 2 S2HIELDB: Mandatory data to collected after PREVENTION activation
| Date: | Time of activation/initial evaluation:/ | Age and sex: | |
|---|---|---|---|
| Allergies: Yes/No | Days in hospital: ____ | Days symptomatic in hospital/at home: ____ | |
| Signs and symptoms | History | ||
|
Signs
Systolic blood pressure: ____ Heart rate: ____ O2 saturation: ____ Symptoms Assess DVT: Lower limb pain: Yes/No Swelling: Yes/No Erythema: Yes/No Homans sign: Yes/No Ollow sign: Yes/No |
Assess PE:
Dyspnea: Yes/No Ischemic like chest pain: Yes/No Near or syncope: Yes/No Cardiac arrest: Yes/No Assess paradoxical embolism: Headache: Yes/No Back pain: Yes/No Abdominal pain: Yes/No Paresthesia: Yes/No |
VTE: Yes/No Obesity: Yes/No Recent infection: Yes/No Puerperium recent: Yes/No Pregnancy: Yes/No Major surgery recent: Yes/No Minor surgery recent: Yes/No Prolonged bed rest/trip: Yes/No Estrogen/OCP use: Yes/No Known active cancer: Yes/No |
|
| Imaging | |||
|
Chest X-ray
Westermark sign: Yes/No PA amputation: Yes/No |
Echocardiogram
RV dilation: Yes/No McConnell sign: Yes/No In-transit thrombus:Yes/No |
CT angiogram
Size: Location: Burden thrombus: |
Lower limb Doppler US Thrombus: Yes/No Location: distal/Proximal Burden Thrombus: Floating thrombus: Yes/No |
| ECG | Laboratory | ||
| Tachycardia: Yes/No Atrial fibrillation or flutter: Yes/No RBBB: Yes/No S1Q3T3: Yes/No |
ST dynamic changes: Yes/No aVR ST elevation: Yes/No V1 qR and ST elevation: Yes/No RV strain overload: Yes/No |
Hemoglobin: ____ Platelets: ____ BNP: ____ D-dimer: ____ High-sensitivity troponin I: ____ eGFR: ___ |
|
| Bleeding risk | |||
| >65-75 years: Yes/No Female: Yes/No BMI < 24 kg/m2: Yes/No Weight < 50-60 kgs: Yes/No Cancer: Yes/No INR > 2.5: Yes/No |
Oral anticoagulation: Yes/No Recent major surgery: Yes/No Uncontrolled hypertension: Yes/No eGFR < 30 ml Liver/kidney disease: Yes/No |
Bleeding predisposition: Yes/No Alcohol abuse: Yes/No Thrombocytopenia: Yes/No History of stroke: Yes/No |
|
DVT: deep venous thrombosis; PE: pulmonary embolism; VTE: venous thromboembolism; OCP: oral contraceptive pills; PA: pulmonary artery; RV: right ventricle; RBBB: right bundle branch block; BNP: B-type natriuretic peptide; eGFR: estimated glomerular filtration rate; INR: international normalized ratio.
After protocol activation, the on-call cardiology fellow will reach out to the PREVENTION-team through an electronic message. The team will be ready to hold an online conference as soon as possible (30 min), providing the on-call fellow enough time to assess the patient and obtain enough data to prove VTE accurately and PE diagnosis, quantify the venous thrombus burden and assess right ventricular dysfunction severity. Finally, within 60-90 min of the initial call, a treatment recommendation will be issued to the physician in charge. The program will follow-up on the clinical condition, treatment response, and in-hospital complications to consistently improve patient care. All information, including clinical data, risk factors, clinical presentation, electrocardiogram (ECG), chest X-ray, biomarkers, diagnosis studies, as well as, therapeutic approach, will be captured in an electronic database. On discharge, patients will have a follow-up in the outpatient clinic if the health-care team deems it necessary.
We considered 60-90 min as a window based on (1) our previous experience14-20, in which we perform stratification, diagnosis, and systemic thrombolysis in the first 90 min after PE patients arrive at the emergency room15; (2) thrombus resistance21, right ventricular ischemia, and myocardial infarction17 are all time-dependent; and finally, (3) evidence from mechanical and pharmacological reperfusion in ST-elevation myocardial infarction and ischemic stroke programs11,22-24. Furthermore, we will activate the cardiac catheter lab and transesophageal echocardiography units in specific cases. According to PERT MGH hospital stratification based on resources and medical specialties9, Hospital Zambrano Hellion has Level 1 PERT; in other words, we have all the resources necessary to carry out a successful program. Recently, the impact of PERT MGH was demonstrated by a significant mortality reduction (25%) in massive PE compared with the previous registries25. This evidence suggests that a rapid response team can modify in-hospital outcomes in a group of patients with high mortality risk.
PREVENTION-team: therapeutic approach
ANTICOAGULATION
The foundation of VTE treatment is anticoagulation, and advanced therapy is the option in impending or clinically unstable patients. Table 311,26,27 shows anticoagulation options in the acute phase, long-term, and extended phase. Unprovoked VTE, recurrence, active cancer, proved or strong suspicion of thrombophilia and a persistently abnormal D-dimer required long-term anticoagulation. In patients with DVT with or without PE, we suggest low-molecular-weight heparin, enoxaparin instead of unfractionated heparin. Furthermore, non-Vitamin K antagonist oral anticoagulants are effective and possess a safer profile compared to Vitamin K antagonists (Table 3). Anticoagulation alone is recommended in low-risk PE patients (clinical stability, no biomarkers expression, without severe right ventricular dysfunction, and moderate thrombus burden); the route of administration regimen and type will be up to the preference of the physicians in charge. In the extended phase, the low-molecular-weight heparin, enoxaparin, is indicated in active cancer patients. Unfractionated heparin is an option in severe kidney diseases, high-risk bleeding, >75 years, hypotension, impending clinical instability patients, and as adjunctive treatment11. We recommend enoxaparin in low-risk PE patients starting with an intravenous bolus, except in elderly patients in whom a dose reduction is mandatory (Table 3)11. Loading apixaban or rivaroxaban doses are an effective and safe option in low-risk PE patients. In intermediate-risk, also called submassive PE, we recommend weight-adjusted unfractionated heparin for the first 24-48 h, over enoxaparin to avoid heparin crossover if clinical status worsens. The use of unfractionated heparin as adjunctive treatment with a posterior switch to enoxaparin is a worldwide recommendation. This regimen was effective and safe, without intracranial hemorrhage in Mexican PE patients submitted to systemic thrombolysis15.
Table 3 Parenteral and oral anticoagulants11,26,36
| Acute phase |
Weight-adjusted unfractionated
heparin
1. Unfractionated heparin 60 U/kg bolus (maximum 4000 U) followed by 12 U/kg infusion (maximum 1000 U) Standard unfractionated heparin regimen 2. Unfractionated heparin 80 U/kg bolus followed by 18 U/kg/h infusion Low-molecular-weight heparin 3. Enoxaparin intravenous bolus 30 mg followed by subcutaneous injection (1 mg/kg BID or 1.5 mg/kg ONCE); in patients > 75 years no bolus and 0.75 mg/kg BID Non-vitamin K antagonist oral anticoagulants (NOACs) 4. Apixaban: 10 mg twice daily for 7 days, followed by 5 mg twice daily 5. Rivaroxaban: 15 mg twice daily for 3 weeks, followed by 20 mg daily |
| Long-term anticoagulation (3-6 months) and Extended treatment (> 6 months) |
Vitamin K antagonists
Warfarin 5 mg daily, overlapped with heparin for first 5 days until two consecutive INR in therapeutic ranges (2-3), and then dose-adjusted to maintain INR 2-3 Low-molecular-weight heparin In patients with active cancer: subcutaneous injection 40 mg ONCE NOACs Dabigatran: 150 mg BID Apixaban: 5 mg or 2.5 mg BID Rivaroxaban: 20 mg or 15 mg ONCE |
NOACs: non-Vitamin K antagonist oral anticoagulants; INR: international normalized ratio.
Advanced therapy
DVT THROMBOLYSIS AND PERCUTANEOUS THROMBECTOMY
Although there are not recommendations to systemic thrombolysis in iliofemoral DVT patients26, we recommend catheter-directed thrombolysis with alteplase at a dose of 0.01 mg/kg/h (maximum 1 mg/h) for iliofemoral DVT (Table 4)11. This therapeutic approach could reduce thrombus burden and venous hypertension, restore venous permeability, rescue limb in case of ischemia, and decrease PE risk. We also recommend percutaneous mechanical or pharmacomechanical thrombolysis. Various percutaneous devices are available with different mechanical principles for the removal of clot or thrombolysis: suction, rotation, rheolytic thrombectomy, and ultrasound28-31. The pharmacoinvasive approach combines the mechanical method and pharmacologic therapy to achieve thrombolysis32. This approach has shown to be effective with a lower dose of the thrombolytic drug and shorter procedural time with no difference in major bleeding or recurrence33. As part of the thrombectomy procedure, we recommend the use of a prophylactic vena cava filter, as 17% of patients treated suffered asymptomatic PE demonstrated on computed tomography scans34. These filters should be removed as soon as possible35. When DVT occurs in the left iliac vein, we encourage the use of intravascular ultrasound to diagnose iliac compression (May-Thurner syndrome)36. If an iliac obstruction, residual thrombus or iliac stenosis is observed, angioplasty and dedicated vein stents use must be considered to improve patency28.
Table 4 Anticoagulation and advanced therapy in venous thromboembolism patients11,26,36,37,38
| Distal deep venous thrombosis |
Anticoagulation
Weight-adjusted unfractionated heparin Unfractionated heparin 60 U/kg bolus (maximum 4000 U) followed by 12 U/kg infusion (maximum 1000 U) Standard unfractionated heparin regimen Unfractionated heparin 80 U/kg bolus followed by 18 U/kg/h infusion Low-molecular-weight heparin Enoxaparin intravenous bolus 30 mg followed by subcutaneous injection (1 mg/kg BID or 1.5 mg/kg ONCE); in patients > 75 years no bolus and 0.75 mg/kg BID Non-Vitamin K antagonist oral anticoagulants (NOACs) Apixaban: 10 mg twice daily for 7 days, followed by 5 mg twice daily Rivaroxaban: 15 mg twice daily for 3 weeks, followed by 20 mg daily |
| Proximal deep venous thrombosis |
Adjunctive treatment
Weight-adjusted unfractionated heparin Unfractionated heparin 60 U/kg bolus (maximum 4000 U) followed by 12 U/kg infusion (maximum 1000 U) Standard unfractionated heparin regimen Unfractionated heparin 80 U/kg bolus followed by 18 U/kg/h infusion Catheter-directed thrombolysis Alteplase, 0.01 mg/kg/h (maximum 1 mg/h) Ultrasound-facilitated catheter-directed thrombolysis (USCDT) |
| Low-risk PE |
Anticoagulation
Weight-adjusted unfractionated heparin 60 U/kg bolus (maximum 4000 U) followed by 12 U/kg infusion (maximum 1000 U) Low-molecular-weight heparin Enoxaparin intravenous bolus 30 mg followed by subcutaneous injection (1 mg/kg BID or 1.5 mg/kg ONCE); in patients > 75 years no bolus and 0.75 mg/kg BID Non-Vitamin K antagonist oral anticoagulants (NOACs) Apixaban: 10 mg twice daily for 7 days, followed by 5 mg twice daily Rivaroxaban: 15 mg twice daily for 3 weeks, followed by 20 mg daily |
| Intermediate risk/submassive PE with or without impending clinical instability |
Weight-adjusted unfractionated
heparin
60 U/kg bolus (maximum 4000 U) followed by 12 U/kg infusion (maximum 1000 U) and close monitoring of blood pressure, oxygen saturation, heart and respiratory rate (consider thrombolysis in case of impending or hypotension or clinical instability) Ultrasound-facilitated catheter-directed thrombolysis (USCDT) USCDT × 2 h with alteplase infusion at 2 mg/h/catheter (range 4-8 mg; 1 vs. 2 lungs) |
| A high-risk or massive PE or submassive PE with impending clinical instability |
Adjunctive treatment
Weight-adjusted unfractionated heparin 60 U/kg bolus (maximum 4000 U) followed by 12 U/kg infusion (maximum 1000 U)/24 h or 48 h followed by enoxaparin 1 mg/kg BID or 1.5 mg/kg ONCE/5 days or apixaban or rivaroxaban Systemic thrombolysis 50 mg of alteplase in 1-2 h in > 60 years 100 mg of alteplase in 1-2 h in < 60 years Weight-adjusted tenecteplase bolus in < 60 years: 30 mg < 60 kg, 35 mg 60-70 kg, 40 mg 70-80 kg, 45 mg 80-90 kg, 50 mg > 90 kg Catheter-directed thrombolysis 30 ± 10 mg of alteplase Pharmacoinvasive approach Thrombus fragmentation with pigtail catheter, 20 mg alteplase infusion in the pulmonary artery and manual or percutaneous aspiration with aspiration device (Aspirex or Pronto) Ultrasound-facilitated catheter-directed thrombolysis (USCDT) USCDT × 2 h with alteplase infusion at 2 mg/h/catheter (range 4-8 mg; 1 vs. 2 lungs) |
| Absolute contraindication for anticoagulation or thrombolysis |
Venous cava filter
Removed temporary filters between day 24 and 54 after placement. |
PE THROMBOLYSIS
International and national guidelines26,27-38 recommend unfractionated heparin as adjunctive treatment and systemic thrombolysis in a well-selected (Table 5)11 high-risk or massive PE patient (IIb). European and American College of Chest Physicians27,37 recommendations are against thrombolysis in intermediate high-risk or submassive PE patients because of the increased rate of intracranial hemorrhage39. The PEITHO study40 and additional previous evidence have shown in-hospital improvement outcome, with systemic thrombolysis14,16,17,19 in this group. Considering current and previous evidence, we recommended weight-adjusted unfractionated heparin as adjunctive treatment and systemic thrombolysis (IIB) in a well-selected high-risk or massive PE patient. We recommend half dose short-term alteplase infusion (Table 4), instead tenecteplase in patients over 60 years considering the high incidence of intracranial hemorrhages, especially in female patients. At present, to the best of our knowledge, half dose short-term alteplase infusion has no evidence of intracranial hemorrhage in the elderly population40. Furthermore, we recommend 1 or 2 h 100 mg alteplase infusion or tenecteplase in a bolus in patients <60 years. Avoid unnecessary venous or arterial punctures to reduce major or minor bleeding complications. Systemic thrombolysis would be an important therapeutic option in intermediate-high-risk or submassive PE with impending clinical instability11 defined with at least one: oxygen desaturation <90%, respiratory distress, blood pressure in lower limits, advanced degree right branch block, severe global right ventricular hypokinesis, tricuspid annular plane systolic excursion <13 mm, high measurements of cardiac I troponin high-sensitivity, and B-type natriuretic peptide.
Table 5 Absolute contraindications for thrombolysis11
| Previous intracranial hemorrhage
Structural cerebrovascular disease Intracranial malignant neoplasm Active bleeding (especially gastrointestinal in last 30 day |
Aortic dissection or suspicion of
Recent cranial surgery or facial trauma with evidence of fracture or cerebral lesion INR > 2.5 Stroke in past 3 months |
INR: international normalized ratio.
We recommend pharmacoinvasive therapy in patients with intermediate- or high-risk bleeding complications since this therapeutic approach showed efficacy and safety in the Mexican population41. Recently, the OPTALYSE trial42 significantly reduced alteplase dose and procedure time compared with previous ultrasound-facilitated catheter-directed thrombolysis studies43,44 (Table 6). The OPTALYSE approach improves the inadmissible long-term infusions (~12 h) in cardiogenic shock or low cardiac output syndrome patients through a low-dose ultrasound-facilitated catheter-directed thrombolysis. Although alteplase 2 mg in 2-h short infusion had no major bleeding complications in a broad clinical PE spectrum, including submassive PE patients, we will recommend 4 mg to obtain a better reperfusion42 (Table 4). Finally, we recommend temporary inferior vena cava filters in patients with absolute contraindications for anticoagulation and thrombolysis in probed proximal DVT with or without in-transit thrombus patients11 (Table 4).
Table 6 Therapeutic alternatives in high-risk bleeding patients11,42
| Low-dose catheter-directed thrombolysis
(alteplase 20-40 mg) OPTALYSE study: treatment arm 1 (alteplase 2 mg/lung/2 h) and treatment arm 2 (alteplase 2 mg/lung/4 h) |
Invasive pharmacological treatment with thrombi
fragmentation and aspiration Surgical embolectomy Vena cava filters |
Patent foramen oval (PFO) and clinical or subclinical paradoxical cerebral or systemic emboli are frequent and an underestimated complication in submassive and massive PE patients45. Transesophageal echocardiogram identifies a high incidence of PFO (56%) and cerebral magnetic resonance a high incidence (17%) of subclinical ischemic stroke-related with a large shunt in submassive PE patients. Hemorrhagic transformation of subclinical ischemic stroke45 could explain unexpected intracranial hemorrhages after anticoagulation alone or advanced therapy in PE patients. Transthoracic echocardiogram with peripheral intravenous agitated saline bubbles to screen for PFO is mandatory45. PREVENTION-team should look for symptoms or signs suggesting central or systemic embolism in the clinical evaluation of high-clinical suspicion PE patients (Table 2).
Research and educational activities
Members of the core team will be the steering committee for all PREVENTION-team activities. Secondary objectives include leading research protocols, creation of support groups, and expand our network. We will set into an online database that will store all information regarding demographics, clinical presentation, therapeutic and diagnosis approaches, as well as the overall outcome for further research and analysis. Expansion of the network will allow us to implement our system in another clinical setting, identify possible loopholes not evident at our hospital, and further improve awareness of VTE. The creation of support groups creates a feeling of identification among patients, improving their well-being and health care. The PREVENTION-team will hold monthly meetings to review protocol activations, assess the response of the team, and troubleshoot and address any system issues. Educational activities, such as clinical case presentations and discussions, teaching sessions, and case simulations, will be important to maintain program quality.
Although the main target of the program is an optimal fast-track treatment in VTE patients, primary or secondary prevention to reduce incidence or recurrence will be mandatory. We have had in-hospital strategies (thrombosis-free hospital), such as thrombosis risk stratification and pharmacologic and no-pharmacologic primary prevention to reduce VTE events for many years; however, these kinds of strategies lack at home. We identified that over 70% of PE patients come from a hospital outside. Thus, we propose patient and family education to stimulate early VTE recognition, identify trigger factors, and implement secondary no-pharmacologic prevention to extend the concept of a thrombosis-free hospital to a new concept: a thrombosis-free home.
Discussion
VTE is a major health problem, annually affecting 108 people per 100,00046 and in the United States 300,000-600,00047. The chronic complications of VTE increase mortality, decrease functional class and quality of life, and increase health-care costs. Patients with PTS increase the cost (2-10 billion dollars annually)47 in the United States (7000) and Canada (4527) compared with DVT patients7. PE is the cause of preventable in-hospital and home mortality through pharmacologic or non-pharmacologic primary or secondary prevention. Considering the link between DVT and PE5,6, its high recurrence48, and the high health systems costs7, DVT should not be underestimated48. In the PREVENTION-team, we include the broad clinical spectrum of VTE to perform a fast-track risk stratification, multimodal diagnosis, and treatment to improve PE and DVT patient care. Another important objective will also be to increase the detection of in-hospital and in the emergency room of low-risk PE patients, whose impact and prevalence are not well defined.
At present, national and international guidelines lack the strong class of recommendation and level of evidence (IA) and do not yet consider new therapeutic approaches11,26,27. Furthermore, there is an underuse of systemic thrombolysis even in high-risk PE due to a fear of bleeding complications, and we do not have any evidence or recommendations in octogenarian and nonagenarian patients. Although systemic thrombolysis is a dark zone in intermediate high-risk or submassive PE patients, those with impending clinical instability should be eligible. In addition, treatment decision-making more often is based on personal medical experience9 instead of consensus discussion among experts. We hope that PREVENTION-team unifies risk stratification, diagnosis, and therapeutic approach through a coordinated action among health system staff to improve the quality of VTE patient care in our hospital.
The first “Mexican PERT”
In 1993, we performed the first successful systemic thrombolysis in a massive PE patient in Mexico17, following the lesson from the first national program on systemic fibrinolysis in ST-elevation myocardial infarction in the emergency room at Cardiology Hospital of the National Medical Center, IMSS. Shortly after that, we launched a successful open-label, randomized control trial proving that short-term streptokinase infusion by peripheral vein compared with unfractionated heparin reduces mortality in cardiogenic shock and massive PE patients19. The emergency, nuclear medicine, and echocardiography teams were activated quickly and efficiently, and systemic thrombolysis was delivered in the first 90 min after patient arrival at the emergency room department. The next challenge was to reproduce this approach 24 h a day, 365 days a year. All emergency physicians received training in echocardiography to improve patient care, and in the case of V/Q lung scan unavailability, in high clinical suspicion patients with clinical, ECG, and echocardiographic findings of severe pulmonary hypertension and right ventricular dysfunction with impending clinical instability or hypotension, an experienced physician administered thrombolysis by peripheral vein. Following this strategy, we perform successful systemic thrombolysis in 11 PE patients16. European Cardiology Society guidelines provide for this approach IC evidence level27.
In another hand, the process was very slow and complicated in submassive PE patients. In this group, although echocardiogram aided in the evaluation of the right ventricular function, the support of the departments of echocardiography and nuclear medicine had delays of up to 24 h16. Hence, we launched a working group, including physicians of the emergency room department and heads of echocardiography and nuclear medicine departments. After several meetings, we were able to agree in a significant reduction (90 min) of the time needed to perform the studies. Eight years later, in 2009, we perform our second 24 h a day, 365 days a year fast-track program for thrombolysis in submassive, impending clinical instability, and massive PE patients15. In this period, we perform under high clinical suspicion, ECG, and echocardiography findings successful thrombolysis in 8 PE patients. The time to perform risk stratification, diagnosis, and systemic thrombolysis treatment were around 60 min in submassive and massive PE15.
The need to improve PE patient care
Nineteen years after our fast-track programs for thrombolysis in massive19 and submassive16 PE patients, the first formal PERT9 emerged as a priority need to deliver rapid assessment and treatment of patients whose clinical condition is deteriorating but are not yet in shock or cardiac arrest49. The PERT consortium was then officially inaugurated in 2015, to create a sense of community where the different PERT programs across the world could share their experiences and work together and improve patient care. The design of our program is based on the already existing protocols and in our 24 years of experience14-20. We add some characteristics obtained from our experience with the entire clinical spectrum of VTE in mind what increases the quality of the program. With the implementation of the PREVENTION-team program, we look to provide fast, efficient, and time-saving treatment, potentially preserving lives and reducing bleeding and chronic complications in VTE patients. Furthermore, we will try to increase detection of low-risk PE. The success of the program will be the rapid and efficient communication among paramedic staff, technicians, residents, fellows, medical students, and physicians. Furthermore, the proper layout, functioning, and usage of the resources will be determinant in protocol success.
Finally, the activation of the PREVENTION-team program will pave the way for research and educational activities, including grand rounds, innovation projects, teaching sessions, and case discussion. Furthermore, another objective is to inspire consciousness among the hospital community, creating a learning environment and experience for everyone, and increasing the quality program. At present, although we have strategies including risk questionnaires at admission, compression stockings, or primary pharmacologic prevention to prevent in-hospital VTE, preventive strategies are lacking at home for patients with the same VTE high-risk. Finally, considering these observations, it is mandatory to extend the concept of the thrombosis-free hospital to the thrombosis-free home.
VTE is a very common cardiovascular disease with high morbidity and mortality, increased health-care costs, and complex treatment. Current VTE treatment is not standardized and depends on individual decisions of several medical specialties related to patient care (pulmonologists, cardiologists, internal medicine, surgeons, etc.). In addition, to the best of our knowledge, there are no available fast-track advanced programs in Mexico. Furthermore, international and national guidelines for PE management11,26,27 do not include a specific door-to needle timeframe to initiate advanced therapy, even though right ventricular ischemia and thrombus resistance are time dependent. Therefore, it is imperative to improve health care through an expert-conformed multidisciplinary team to fast-track stratification and optimal treatment to improve the outcome and long-term complications of VTE patients. PREVENTION-team links a group of physicians with different areas of expertise, guaranteeing collective, and synchronized medical care. We hope to create a strong network and inspire physicians, fellows, residents, nurses, medical students and staff of our hospital and health system not only to be aware of the problem but also enrich themselves with the necessary tools to diagnose and offer the best treatment possible.











 nueva página del texto (beta)
nueva página del texto (beta)