Introduction and objective
Second-generation metallic drug-eluting stents (DESs) have become the first-line devices in percutaneous coronary intervention (PCI) thanks to lower rates of target lesion revascularization (TLR), stent thrombosis (ST), and major adverse cardiac events (MACE) when compared to simple angioplasty and bare metal stents1. Nevertheless, permanent caging of the vessel represents their main drawback. Bioresorbable vascular scaffolds (BVSs) appeared more than 10 years ago to avoid this problem. The first approved BVS was the Absorb® bioresorbable scaffolds (BRS) (Abbot Vascular, Santa Clara, California, USA) with an expected time to backbone resorption between 2 and 3 years due to PPLA hydrolysis2. To reduce this resorption process, Magmaris® scaffold (Biotronik AG, Bulach, Switzerland) was designed as the first non-polymeric scaffold, with a magnesium alloy backbone that can be completely degraded by 9-12 months after PCI3. Optimal expansion and apposition, with no significant scaffold disruption, have been demonstrated for Absorb® BVS immediately after PCI4; however, there is few evidence regarding Magmaris® acute performance after PCI.
Materials and methods
Study design and patient population
This study wants to evaluate the mechanical properties and performance of Magmaris® scaffold at baseline (immediately after PCI) in comparison to the most studied BVS: the Absorb 1.1® (Abbot Vascular, Santa Clara, California, USA). Within the global pool of patients admitted to PCI in our cath lab between November 2016 and October 2017, we looked for those who could benefit the most from metallic BRS5. According to this, 10 coronary lesions were treated with Magmaris® device in 10 different patients. Lesions considered as suitable for Magmaris® deployment included: de novo coronary lesions with a diameter between 2.5 and 3.5 mm and with none/mild calcification. Bifurcation lesions were also admitted, and there were no restrictions regarding PCI indication: stable angina and acute coronary syndrome were admitted. Left main disease (left main coronary artery disease [LMCD]) and ostial lesions were excluded, as well as chronic total occlusions or in-stent restenosis.
The clinical exclusion criteria included age > 75 years old, history or high risk of bleeding, heparin or antiplatelet treatments intolerance, and expected survival < 1 year.
On the other hand, 10 patients with 10 coronary lesions who had undergone PCI with at least one Absorb 1.1® BVS represented the control group. They were selected in a retrospective, blinded, non-randomized way from the total cohort of patients treated with Absorb® who had undergone intracoronary optical coherence tomography (OCT) evaluation at baseline. All indications for PCI had been admitted. The only angiographic exclusion criteria for this group had been: LMCD and lesions with diameters < 2.5 mm or > 4 mm. The aforementioned clinical exclusion criteria also applied to this group with the only exception of age.
Informed written consent was obtained in all cases.
Study devices
Ten coronary lesions were treated with Magmaris® scaffold and 10 lesions with Absorb 1.1® BVS. Even though Absorb® and Magmaris® are both of them BRS, important differences between their conformation and behavior must be highlighted. Magmaris® scaffold is the only available metallic BRS with CE approval. Its magnesium alloy backbone is completely coated by a bioresorbable polymeric layer of poly-L-lactic acid (PLLA) from which sirolimus antiproliferative drug is released6. The strut thickness is 150 µm5. On the contrary, Absorb® BVS is an everolimus-eluting polymeric BRS, with a PLLA backbone covered by a poy-D-lactic acid coating2. Absorb® strut thickness accounts to 156 µm2. Different mechanical properties have been described for these devices as, for example, higher tensile strength and elongation-to-break for Magmaris®5. Nevertheless, the main difference between them is the expected time to completely scaffold resorption: 9-12 months for Magmaris®3,6 and 2-3 years for Absorb® BVS2.
Procedure and OCT analyses
In our study, predilatation was mandatory for both groups. Semi-compliant balloons were used in a 1:1 balloon/artery relationship to warrant an optimal preparation of the lesion. Between all the different available diameters and lengths for each device, the operator decided the most appropriate size in each case according to visual and OCT assessment. Scaffold overlapping was allowed if necessary to warrant a completely coverage of the lesion. Per protocol, high-pressure postdilatation was mandatory after scaffold deployment in both Absorb® and Magmaris® groups. Non-compliant balloons were used in 1:1 balloon/scaffold relationship, with a minimum inflation pressure of 16 atmospheres (atm).
OCT intracoronary analyses were performed with the Lunawave Coronary console® and the Fastview Catheter® (Terumo Corp., Tokyo, Japan). In all cases, OCT evaluation was done after lesion predilatation and both after scaffold deployment and postdilatation to warrant: (a) the best evaluation of the lesion, (b) appropriate size of the device, and (c) optimal scaffold expansion and apposition. Images acquisition technique has been previously described4.
Quantitative OCT analyses were done with the offline software provided by Terumo® (Terumo Corp., Tokyo, Japan). In both groups, we measured frame by frame all the scaffold segment of the vessel. Lumen, scaffold, and vessel diameters were measured, and malapposed and disrupted struts were also identified. Definitions and analysis methods have been previously published, and they were applied in both the Absorb® and Magmaris® groups4,6.
All procedures were performed from a radial approach and under unfractionated heparin treatment. Dual antiplatelet therapy with aspirin and a P2Y12 inhibitor was recommended according to current guidelines for a minimum of 12 months7.
Study endpoints
PRIMARY ENDPOINT
Evaluation of acute effectiveness and safety of two different BVSs (Absorb 1.1® and Magmaris®) regarding OCT postprocedure evaluation of lumen, scaffold, and vessel diameters, as well as percentage of struts disruption and malapposition.
SECONDARY ENDPOINTS INCLUDE
Procedural success rate (defined as the achievement of a residual stenosis < 20% in the absence of death, myocardial infarction [MI], or TLR during in-hospital stay), and MACE rate at 30-day follow-up (defined as the combination of cardiac death, target vessel-related MI, and clinically driven TLR).
Statistical analysis
Continuous variables with a normal distribution are presented as mean and standard deviations, while categorical variables are presented as percentages. Unpaired t-test (in case of parametric distribution) or MannWhitney U-test (in case of non-parametric distribution) was used to compare continuous variables between groups. Chi-square test or Fishers exact test was used to assess significance associations for categorical variables. A p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 21.
Results
A total of 20 patients (20 lesions) were included in this study. Eighteen Absorb® scaffolds were deployed for the treatment of 10 lesions between June and October 2015, and 10 lesions received 17 Magmaris® devices between November 2016 and October 2017. Baseline clinical characteristics were well balanced between groups (Table 1). Most patients were men, with age ranged between 55 and 65 years and with a diagnosis of hypertension (60% vs. 70%, non-significant [NS]) and dyslipidemia (90% vs. 70% NS). No patients suffered from anemia or chronic kidney disease.
Table 1 Baseline patients characteristics
| PLLA BVS group | Mg scaffold group | p value | |
|---|---|---|---|
| n: 10 (100%) | n: 10 (100%) | ||
| Age (years) | 56.79 ± 11.38 | 65.35 ± 9.75 | 0.45 |
| Women | 2 (20) | 3 (30) | 0.38 |
| Current smokers | 4 (40) | 5 (50) | 0.37 |
| Ex-smokers | 3 (30) | 3 (30) | NA |
| Non-smokers | 3 (30) | 2 (20) | 0.38 |
| Arterial hypertension | 6 (60) | 7 (70) | 0.45 |
| Dislypemia | 9 (90) | 7 (70) | > 0.45 |
| DM on AOD | 3 (30) | 4 (40) | Ø 0.45 |
| DM on insulin | 1 (10) | 0 | 0.09 |
| Body mass index | |||
| < 25 kg/m2 | 0 | 0 | NA |
| 25-29.9 kg/m2 | 5 (50) | 4 (40) | 0.37 |
| ≥ 30 kg/m2 | 5 (50) | 6 (60) | 0.25 |
| Previous stroke | 0 | 0 | NA |
| Peripheral artery disease | 2 (20) | 2 (20) | NA |
| Atrial fibrillation | 0 | 0 | NA |
| Previous coronary artery disease | 1 (10) | 1 (10) | NA |
| Previous target vessel revascularization | 0 | 0 | NA |
| Current indication for PCI | |||
| Effort angina | 7 (70) | 6 (60) | > 0.45 |
| ACS without ST segment elevation | 3 (30) | 4 (40) | 0.45 |
| STEMI | 0 | 0 | NA |
| HbA1c (%) | 6.05 ± 0.99 | 6.99 ± 0.78 | 0.28 |
| Haemoglobine g/dL | 14.72 ± 0.82 | 14.43 ± 0.35 | 0.54 |
| Platelets × 103/µL | 270.07 ± 93.4 | 253.52 ± 85.30 | 0.21 |
| Creatinine mg/dl | 0.77 ± 0.16 | 0.81 ± 0.21 | 0.24 |
| Mean eGFR mL/min/1.73 m2 | 112.31 ± 37.48 | 109.91 ± 41.3 | 0.17 |
| Minimun eGFR | 80.78 | 79.3 | 0.43 |
Values are n (%) or mean ± standard deviation; *NA: not applicable. ACS: acute coronary syndrome; AOD: antidiabetic oral drugs; DM: Diabetes Mellitus; xeGFR: estimated glomerular filtration rate; STEMI: ST-elevation myocardial infarction; HbA1c: hemoglobin A1C; BVS: bioresorbable vascular scaffolds; PLLA: poly-L-lactic acid.
The most common indication for PCI was effort angina in both groups (70% vs. 60% NS) with no ST-elevation MI cases included. Main target vessel was left anterior descendent artery in both Absorb® (70%) and Magmaris® group (80%), with a mean vessel diameter of 3.46 ± 0.23 and 3.52 ± 0.19mm, p = 0.56, respectively. According to the American Heart Association classification, the most lesions were identified as moderate/high-risk lesions: 40% versus 60%, p = 0.37 were type B and 40% versus 30%, p = 0.45 were type C lesions in Absorb® and Magmaris® group, respectively. No statistically significant differences between groups were identified regarding to angiographic and procedural characteristics (Table 2) unless slightly higher postdilatation pressures for Magmaris® devices (18.01 ± 2.15 vs. 17.20 ± 3.80 atm, p = 0.05). Procedural success rate was 100%. Postprocedural OCT findings are presented in Table 3. We analyzed 21,016 PLLA struts and 20,584 magnesium struts in each group. Both mean scaffold and vessel diameters were significantly larger in Magmaris® group: 3.11 ± 0.38 versus 3.07 ± 0.36 mm, p = 0.03 and 4.12 ± 0.51 versus 4.04 ± 0.46mm, p = 0.04; even in the presence of higher plaque burden (mean plaque area was 6.17 ± 1.79 mm2 in Magmaris® group versus 6.07 ± 1.28 mm2 in Absorb® group, p = 0.02). Low rates of malapposition and acute scaffold disruption were demonstrated for both devices after high pressure postdilatation; nevertheless, we identified significantly lower percentages of malapposed (1.06% vs. 1.46 %, p = 0.01) and disrupted struts (0.15% vs. 0.27 %, p = 0.03) in Magmaris® group. All patients completed 30-day follow-up with no cardiac death, target vessel-related MI, or clinically-driven TLR reported.
Table 2 Angiographic lesion characteristics and procedural aspects
| PLLA BVS group | Mg scaffold group | p value | |
|---|---|---|---|
| Angiographic lesion characteristic (%) | 10 (100) | 10 (100) | |
| Treated artery | |||
| Left anterior descendent artery | 7 (70) | 8 (80) | 0.39 |
| Right coronary artery | 3 (30) | 2 (20) | 0.38 |
| AHA lesion classification | |||
| A | 2 (20) | 1 (10) | 0.39 |
| B | 4 (40) | 6 (60) | 0.37 |
| C | 4 (40) | 3 (30) | 0.45 |
| Bifurcations | 1 (10) | 1 (10) | NA |
| Chronic total occlusions | 0 | 0 | NA |
| Thrombus | 2 (20) | 1 (10) | 0.39 |
| Mean vessel diameter mm | 3.46 ± 0.23 | 3.52 ± 0.19 | 0.56 |
| Procedural aspects | |||
| Predilatation | 10 (100) | 10 (100) | NA |
| Mean predilatation balloon diameter mm | 3.2 ± 0.36 | 3.39 ± 0.21 | 0.52 |
| Mean predilatation balloon length mm | 19.2 ± 4.37 | 19.4 ± 3.2 | 0.67 |
| Mean predilation balloon pressure atm | 12.4 ± 3.04 | 13.2 ± 2.9 | 0.49 |
| Mean number of BVS deployed per lesion | 1.79 | 1.63 | 0.23 |
| Mean BVS diameter mm | 3.22 ± 0.32 | 3.27 ± 0.28 | 0.21 |
| Mean BVS length mm | 22.5 ± 5.34 | 21.7 ± 6.16 | 0.41 |
| Mean total length scaffold per lesion mm | 35.21 ± 19.25 | 32.19 ± 15.38 | 0.09 |
| Mean pressure used in BVS deployment atm | 14.08 ± 2.73 | 14.98 ± 3.10 | 0.21 |
| Postdilatation | 10 (100) | 10 (100) | NA |
| Mean postdilatation balloons diameter mm | 3.5 ± 0.32 | 3.49 ± 0.39 | 0.23 |
| Mean postdilatation balloons length mm | 13.09 ± 3.55 | 13.78 ± 2.25 | 0.19 |
| Mean postdilatation balloon pressure atm | 17.20 ± 3.80 | 18.01 ± 2.15 | 0.05* |
| Maximum postdilatation balloon pressure atm | 20.00 ± 3.80 | 21.00 ± 3.75 | 0.04* |
| Postdilatation balloon/scaffold diameter ratio | 1.01 | 1.02 | 0.19 |
| Pre-PCI TIMI flow | |||
| 0/I | 0 | 0 | NA |
| II | 3 (30) | 1 (10) | 0.44 |
| III | 7 (70) | 9 (90) | > 0.45 |
| Post-PCI TIMI III flow | 10 (100) | 10 (100) | NA |
Values are n (%) or mean ± standard deviation.
*NA: not applicable.
BVS: bioresorbable vascular scaffold; PCI: percutaneous coronary intervention; PLLA: poly-L-lactic acid; AHA: American Heart Association.
Table 3 Baseline optical coherence tomography findings. Lesion-level and strut-level analyses
| PLLA BVS | Mg scaffold | p value | |
|---|---|---|---|
| 18 devices | 17 devices | ||
| Lesion-level analyses | |||
| Mean lumen diameter mm | 2.91 ± 0.38 | 2.99 ± 0.91 | 0.08 |
| Minimal lumen diameter mm | 2.70 ± 0.73 | 2.73 ± 0.76 | 0.07 |
| Maximal lumen diameter mm | 3.15 ± 0.41 | 3.22 ± 0.38 | 0.06 |
| Mean vessel diameter mm | 4.04 ± 0.46 | 4.12 ± 0.51 | 0.04 |
| Minimal vessel diameter mm | 3.87 ± 0.42 | 3.91 ± 0.48 | 0.05 |
| Maximal vessel diameter mm | 4.18 ± 0.47 | 4.23 ± 0.60 | 0.03 |
| Mean scaffold diameter mm | 3.07 ± 0.36 | 3.11 ± 0.38 | 0.03 |
| Minimal scaffold diameter mm | 2.86 ± 0.42 | 2.90 ± 0.70 | 0.02 |
| Maximal scaffold diameter mm | 3.30 ± 0.36 | 3.38 ± 0.46 | 0.03 |
| Mean plaque area mm2 | 6.07 ± 1.28 | 6.17 ± 1.79 | 0.02 |
| Mean eccentricity index | 0.13 ± 0.05 | 0.09 ± 0.01 | 0.02 |
| Strut-level analyses | |||
| Number of struts analyzed | 21,016 | 20,584 | 0.29 |
| % of malapposed struts | 1.46 | 1.06 | 0.01 |
| Total number of malapposed struts | 308 | 219 | |
| % of disrupted struts | 0.27 | 0.15 | 0.03 |
| Total number of disrupted struts | 58 | 31 |
Values are n (%) or mean ± standard deviation.
PLLA: poly-L-lactic acid; BVS: bioresorbable vascular scaffold.
Discussion
In this study, we evaluated postprocedural performance of Magmaris® scaffold in comparison to the most studied to the date BVS: the Absorb 1.1® (Abbot Vascular, Santa Clara, California, USA). The chief findings are as follows: (a) there is no class effect regarding acute device performance between metallic and polymeric BRS, (b) higher scaffold and vessel diameters can be achieved with Magmaris® device in comparison to same size Absorb® BVS, (c) significantly higher percentage of elongation-to-break for Magmaris® device allows the operator to achieve higher scaffold and vessel diameters in a safe manner, with lower rates of acute scaffold disruption, and (d) for the same reason, high-pressure postdilatation has been demonstrated to be safe and useful as it reduces malapposition rates.
BVS class effect has been suggested due to their common resorbable nature and structural features, such as wide struts thickness (150 µm approximately)2,5,8. Absorb® and Desolve® are the polymeric scaffolds meanwhile Magmaris® is the only metallic BRS. Mattesini et al.9 demonstrated comparable results in terms of mean lumen and scaffold area when analyzing postdeployment OCT evaluations for both polymeric devices, so similar acute mechanical properties have been suggested for both Absorb® and Desolve®. However, do these results also apply to metallic BRS? A time-dependent recoil phenomenon as well as higher rates of acute recoil has been demonstrated for polymeric BVS when compared to Magmaris® in preclinical studies10. We report the first in vivo comparison of acute mechanical performance between Magmaris® and Absorb® devices. Significantly, larger vessel and scaffold diameters were demonstrated in Magmaris® group when comparing to a well-balanced cohort of patients treated with Absorb® (4.12 ± 0.51 vs. 4.04 ± 0.46 mm, p = 0.04 and 3.11 ± 0.38 vs. 3.07 ± 0.36 mm, p = 0.03, respectively). As there were significant differences neither in lesion characteristics nor in procedural aspects between groups, these results suggest higher expansion and radial force for Magmaris®. Furthermore, lower eccentricity index (0.09 ± 0.01 vs. 0.13 ± 0.05, p = 0.02) supports the idea of a better geometrical adaptation to the vessel wall for magnesium BVS, which could be explained thanks to its lower bending stiffness and higher flexibility10.
In addition to this, Magmaris® greater percentage of elongation-at-break had been hypothesized attending to mechanical properties of magnesium alloy11. Nevertheless, no clinical evidence was available. We evaluated for the first time in vivo acute scaffold disruption of Magmaris® device by OCT intracoronary imaging (Fig. 1). The percentage of disrupted struts in Magmaris® group was minimal and significantly lower than in the Absorb® group (0.15% vs. 0.27%, p = 0.03). These results confirm metallic BRS higher resistance to rupture, even when postdilatated at higher pressure levels (18.01 ± 2.15 vs. 17.20 ± 3.80 atm, p = 0.05).
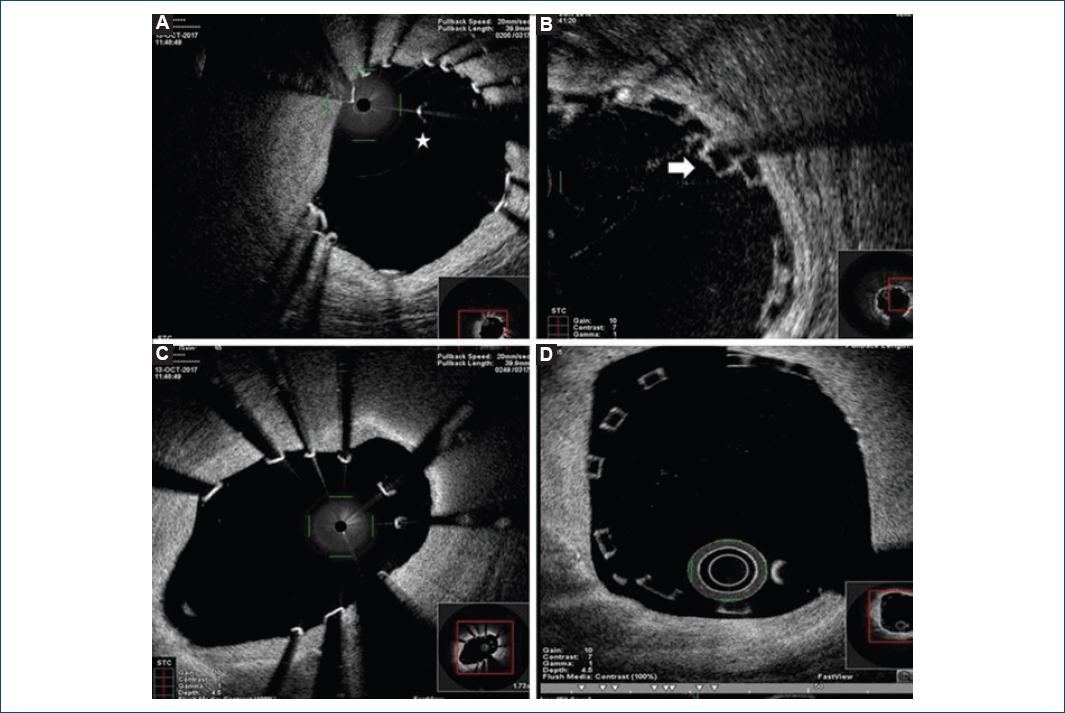
Figure 1 Optical coherence tomography (OCT) in vivo evaluation of Magmaris® and Absorb® scaffolds disruption and malapposition. 1A and 1B: show different OCT intracoronary images of disrupted struts: the star points to a magnesium isolated strut and the arrow a stacked polymeric disrupted strut. Meanwhile, images 1C and 1D show metallic (1C) and polymeric (1D) bioresorbable scaffolds malapposition.
Main concern about polymeric scaffolds comes from their original slightly higher rates of ST when comparing to second-generation DES12,13. Nevertheless, the application of the PSP strategy (including high-pressure postdilatation) has demonstrated a significant reduction in ST and MACE rates after BVS scaffolding with the Absorb® device14,15, with no higher rates of acute scaffold disruption4. In line with this, we decided to perform and evaluate high-pressure postdilatation per protocol in all lesions treated with Magmaris® in our cath lab. We confirmed that a greater percentage of elongation-at-break allowed the operator to reduce Magmaris® malapposition rates in a safe manner (1.06% vs. 1.46 %, p = 0.01 of malapposed struts in Magmaris® and Absorb® groups, respectively). Even though Magmaris® device has been suggested to have lower acute thrombogenicity16 with no reported cases of ST6,17,18, it is well known that malapposition and infraexpansion significantly increase the risk of scaffold thrombosis and restenosis. According to this, we support the use of high-pressure postdilatation after magnesium-scaffold deployment to optimize angiographic and secondary clinical results, specially avoiding scaffold malapposition (Fig. 1).
In conclusion, this first comparative study between Absorb® and Magmaris® devices supports the use of a PSP strategy for both scaffolds deployment. Optimal preparation of the lesion joined to appropriate sizing of the scaffold and high-pressure postdilatation reduces scaffold infraexpansion and malapposition, without acute security concerns. However, slight differences in acute mechanical performance between both devices have also been demonstrated, refusing a common class effect for all BVS.
Limitations
Main limitation of our study comes from the small number of patients included. However, we want to highlight that this study is the one which includes the highest number of struts analyzed for Magmaris® device after deployment (20584 struts vs. only 195.67 struts in Biosolve II trial6). Moreover, the date is the only study which compares Absorb® versus Magmaris® scaffolds. We are also aware of limitations derived from the non-randomized, observational nature of the study, as well as the possible bias generated by patients selection. Nevertheless, we would like to emphasize patients/lesions included represent the target population for these devices. More evidence is needed to confirm our findings in a larger population, as well as to complete shortlong-term clinical follow-up.











 text new page (beta)
text new page (beta)


